BISAC NAT010000 Ecology
BISAC NAT045050 Ecosystems & Habitats / Coastal Regions & Shorelines
BISAC NAT025000 Ecosystems & Habitats / Oceans & Seas
BISAC NAT045030 Ecosystems & Habitats / Polar Regions
BISAC SCI081000 Earth Sciences / Hydrology
BISAC SCI092000 Global Warming & Climate Change
BISAC SCI020000 Life Sciences / Ecology
BISAC SCI039000 Life Sciences / Marine Biology
BISAC SOC053000 Regional Studies
BISAC TEC060000 Marine & Naval
Macrofauna is supposed to influence on physic-chemical characteristics of the sea bottom sediments. Through its bioturbation mechanism porosity, area of oxygenated layer and oxygen penetration depth have increased. This lead to alterations in nutrient cycling as well as improvement in redox conditions which define direction of fluxes in the sediments. In oxic conditions phosphorus is transformed into particulate form and thus, its retention and burial increase. In contrary, denitrification is getting weaker and nitrogen returns into the water. The impact of benthic organisms bioirrigation activity on other chemical components in solid sediments is not sufficiently studied. Present investigations were carried out for the most abundant benthic species in the Gulf of Finland Marenzelleria spp. Those polychaetes are active turbators and their irrigation effect lead to significant changes in chemical compounds in the solid sediment. On the basis of statistical analysis of data on vertical distribution of organic carbon content, total iron and manganese in solid sediments and abundance of Marenzelleria spp. there was found that polychaetes have a significant impact on organic carbon content, while for total iron and manganese such regularity is not revealed.
bioturbation, Marenzelleria spp., Gulf of Finland, sediments.
- introduction
Polychaetes of the genus Marenzelleria spp. are invasive species in the Baltic Sea. First observed in the Gulf of Finland in 1990, these organisms have populated wide areas of the bottom and became one of the dominant benthic species [1]. Marenzelleria spp. has a set of characteristics of successful invader: those organisms are euryhaline, can exist in hypoxic waters and withstand hydrogen sulfide environment for a short-time [2, 3]. Physiological characteristics of polychaetes are of particular interest: they burrow into the sediments deeper then indigenous species. The results of laboratory studies and model calculations [4-11] indicate geochemical changes in the sediments and bottom water as a result of bioturbation and irrigation of Marenzelleria spp. Therefore, the main aim of this work is to study the influence of polychaetes activity on the distribution of various chemical characteristics in the solid phase of bottom sediments using statistical analysis of field data observations on organic carbon content, total content of iron and manganese in solid phase of sediments, as well as abundance of Marenzelleria spp. polychaetes.
II. observational data and their analysis
Expedition in the Eastern part of the Gulf of Finland was held in September 2013 and July-August 2015 by Russian State Hydrometeorological University [12]. Map of sampling station is shown in fig. 1.
Fig. 1. Map of sampling stations.
Sediment samples were collected with oceanologic and Borutzky grabs and sliced into 2-2.5 cm thickness layers or thicker. In each layer organic carbon content (Corg) was measured using the carbon express-analyzer AH 7529M, measurement of total iron (SFe) and manganese (SMn) was held on atomic absorption spectrometer “Varian”. Abundance of Marenzelleria spp. in sediments was counted as well. As follows from table 1, abundance of polychaetes in the Eastern part of the Gulf of Finland varied widely during the sampling period.
Table 1. Abundance of Marenzelleria spp. at the stations in the Eastern Gulf of Finland
|
Station/year |
2013 |
2015 |
|
2L |
2928 |
1700 |
|
17F |
1328 |
1120 |
|
2UGMS |
3912 |
1140 |
|
4F |
3784 |
4080 |
|
20F |
240 |
1000 |
|
10F |
1064 |
340 |
|
2F |
|
17020 |
|
8F |
|
620 |
Bold are stations with high Marenzelleria spp. abundance.
For statistical analysis stations within each year were divided into 2 groups: with high and low polychaetes abundance. Average values of organic carbon content, total content of iron and manganese for each group were calculated (table 2). The significance of differences in characteristics in two groups was determined using t-Student test with p<0.05.
Table 2. Average values (± standard deviation) of Corg, SFe and SMn in solid phase of sediments at the stations with high and low Marenzelleria population. Significant differences between characteristics are assigned with asterisk (*p<0.05)
|
Characteristic |
2013 |
2015 |
||
|
High |
Low |
High |
Low |
|
|
Corg |
4.12±0.42* |
5.82±0.60* |
1.78±0.54* |
3.14±0.50* |
|
ΣFe |
4.86±0.56 |
4.85±0.25 |
4.99±0.56 |
5.14±0.49 |
|
ΣMn |
0.14±0.08 |
0.18±0.10 |
0.17±0.08 |
0.16±0.07 |
According to table 2, significant differences were obtained only for organic carbon content, meanwhile in conditions of high Marenzelleria spp. abundance Corg content of the sediments was on average 1.5 times lower due to, apparently, a deeper penetration of oxygen into the sediments because of bioturbation and, consequently, more intense carbon oxidation.
II. cluster analysis of data
As shown above, groups separation on Marenzelleria spp. abundance was carried out according to the "more-less" criterion and distribution of chemical compounds in the sediments was dependent parameter. To identify regularities in Corg, SFe and SMn alteration in the sediments at different stations regardless of polychaetes density cluster analysis is carried out.
Figures 2 and 3 present dendrogram classification of stations according to the content of chemical compounds in the sediments, characteristic of polychaetes abundance was not considered as a parameter in clustering. In both years the station were divided into 3 cluster on carbon content, total content of iron and manganese. The dendrogram shows that the number of classes could be more, but, first, quantity of stations is not large (6 in 2013 and 5 in 2015), and, secondly, there is no need for very small clustering. For each cluster average values of chemical characteristics, as well as number of polychaetes Marenzelleria spp. were calculated (table 3 and 4).
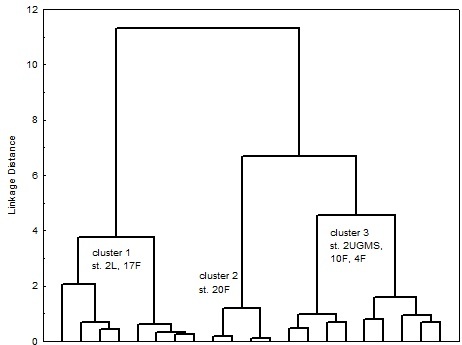
Fig. 2. Dendrogram of station classification for chemical characteristics (Ward’s method) for 2013 year.
Table 3. Average values of Corg, SFe and SMn content in the clusters; Marenzelleria spp. abundance for 2013 year
|
Cluster/characteristic |
1 |
2 |
3 |
|
Corg, % |
5.24* |
5.73* |
3.92 |
|
SFe, % |
4.38* |
5.10 |
5.18 |
|
SMn, % |
0.07* |
0.28* |
0.16 |
|
Marenzelleria abundance, ind./m2 |
moderate 2128 |
low 240 |
high 2920 |
Significant differences between solid phase content of Corg, SFe and SMn compared to 3-d cluster are assigned with asterisk (*p<0.05)
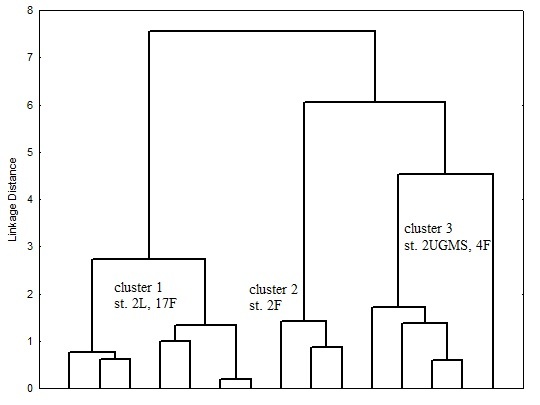
Fig. 3. Dendrogram of station classification for chemical characteristics (Ward’s method) for 2015 year.
Table 4. Average values of Corg, SFe and SMn content in the clusters; Marenzelleria spp. abundance for 2015 year
|
cluster |
1 |
2 |
3 |
|
Corg, % |
3.3* |
1.4 |
2.6* |
|
SFe, % |
4.8 |
4.7 |
5.6* |
|
SMn, % |
0.1 |
0.1 |
0.2 |
|
Marenzelleria abundance, ind./m2 |
low 1400 |
high 17020 |
moderate 2610 |
Significant differences between solid phase content of Corg, SFe and SMn compared to 2-d cluster are assigned with asterisk (*p<0.05)
Results of statistical analysis show that significant differences in average values of chemical compounds were obtained in all clusters only for organic carbon content. The following regularities are observed: stations with low Corg content correspond to high abundance of Marenzelleria spp. polychaetes. Statistically significant differences in SFe and SMn content were obtained only in some cases, however, there was no trend of their changes in dependence of polychaetes population. This may be explained by the fact that the dynamics of iron and manganese content in the sediments is a function of many other characteristics. It is known that the distribution of manganese in sediments depends not only on bioturbation intensity, but benthic flux of Corg and oxygen concentration in overlying water [13]. The formation of iron hydroxides is faster than manganese and it highly dependent on pH [14].
Cluster analysis was also carried out to determine differences in distribution of chemical compounds for 2 years in common. Given the different number of sediments layers at stations and their varying thickness, as well as a significant variability of compounds content, the average values of Corg, SFe and SMn in the upper 4-5 cm layer of sediments were calculated for 10 stations (fig. 4, table 5)
.jpg)
Fig. 4. Dendrogram of station classification for chemical characteristics (Ward’s method) for 2013 and 2015 year.
Clusters 1 and 3 in the dendrogram (fig. 4) were allocated separately due to the fact that at stations 2F (cluster 1) and 20F (cluster 3) in 2015 and 2013, respectively, extreme values of studied characteristics were obtained: the 1st station is characterized by much lower organic carbon content and very high abundance of Marenzelleria spp., the 2nd station is hypoxic and has high content of SMn in addition with very low numbers of benthic organisms. Assessment of difference significance in average values of organic carbon, total iron and manganese contents was obtained for clusters 2 and 4. Significant differences were detected only in total iron and manganese content.
Table 5. Average values of Corg, SFe and SMn content in the clusters; Marenzelleria spp. abundance for 2013 and 2015 years
|
cluster |
1 |
2 |
3 |
4 |
|
Station(s)-year |
2F-2015 |
2L, 17F -2013 2L-2015 |
20F-2013 |
2UGMS, 10F, 4F – 2013, 17F, 4F - 2015 |
|
Corg, % |
1.37 |
4.63 |
5.66 |
3.67 |
|
SFe, % |
4.44 |
4.43* |
5.11 |
5.24* |
|
SMn, % |
0.15 |
0.07* |
0.29 |
0.19* |
|
Marenzelleria spp. abundance, ind./m2 |
high 17020 |
moderate 1985 |
low 240 |
moderate 2792 |
Significant differences between solid phase content of Corg, SFe and SMn are assigned with asterisk (*p<0.05) (t-Student test was made only between clusters 2 and 4 as they contain more than 1 elements)
It can be concluded that intensive bioturbation activity of Marenzelleria spp. in terms of their high abundance leads to decreased organic carbon burial in the upper layer of sediments. Significant impact of polychaetes bioturbation activity on total iron and manganese content was not identified.
IV. Acknowledgment
This work was supported by the grant N 14-50-00095 of the Russian Science Foundation
1. A.A. Maximov, T.R. Eremina, E.K. Lange, L.F. Litvinchuk and O.B. Maximova, “Regime shift in the ecosystem of the eastern Gulf of Finland caused by the invasion of the polychaete Marenzelleria arctia,” Oceanology, vol. 54, pp. 46-53, 2014.
2. A. Bochert, D. Richard and R. Bochert, “Marenzelleria cf.viridis and the sulphide regime,” Aquat. Ecol., vol. 31 (2), pp. 223-231, 1997.
3. D. Schiedek, “Marenzelleria cf. viridis (Polychaeta: Spionidae) - ecophysiological adaptations to a life in the coastal waters of the Baltic Sea,” Aquat. Ecol., vol. 31, pp. 199-210, 1997.
4. S. Hietanen, A. O. Laine, K. Lukkari, “The complex effects of the invasive polychaetes Marenzelleria spp. on benthic nutrient dynamics,” J. of experimental Marine Biology and Ecology, N 352, pp. 89-102, 2007.
5. S. Viitasalo-Frösén, A.O. Laine, M. Lehtiniemi, “Habitat modification mediated by motile surface stirrers versus semi-motile burrowers: potential for a positive feedback mechanism in a eutrophied ecosystem,” Mar. Ecol. Prog. Ser., N 376, pp. 21-32, 2009.
6. C.O. Quintana, T. Hansen, M. Delefosse, G. Banta, E. Kristensen, “Burrow ventilation and associated porewater irrigation by the polychaete Marenzelleria viridis,” Exp. Mar. Biol. Ecol., N 397, pp. 179-187, 2011.
7. E. Kristensen, T. Hansen, M. Delefosse, G.T. Banta, C.O. Quintana, “Contrasting effects of the polychaete Marenzelleria viridis and Nereis diversicolor on benthic metabolism and solute transport in sandy coastal sediment,” Mar. Ecol. Prog. Ser., N 425, pp. 125-139, 2011.
8. J. Norkko et al., “A welcome can of worms? Hypoxia mitigation by an invasive species,” Glob. Change Biol., vol. 18, N 2, pp. 422-434, 2012.
9. J. R. Renz and S. Forster, “Are similar worms different? A comparative tracer study on bioturbation in the three sibling species Marenzelleria arctia, M. viridis, and M. neglecta from the Baltic Sea,” Limnol. and Oceanogr., vol. 58, pp. 20462058, 2013.
10. E.V. Voloshchuk, T.R. Eremina, V.A. Ryabchenko, “Modeling of biogeochemical processes in the sediments of the eastern part of the Gulf of Finland by means of diagenetic model,” Fundam. I prikl. Gidrofizika, vol. 8, pp. 106-113, 2015.
11. N. Ekeroth, S. Blomqvist, P.O. J. Hall, “Nutrient fluxes from reduced Baltic Sea sediment: effects of oxygenation and macrobenthos,” Mar. Ecol. Prog. Ser., N 544, pp. 77-92, 2016.
12. T. R. Eremina, E. V. Voloshchuk, A. A. Maximov, “Assessment of biogeochemical changes in the sediments of the Eastern part of the Gulf of Finland due to invasion of polychaetes Marenzelleria spp. on observational data and modeling results,” Izvestiya RGO, vol. 148, N 1, pp. 55-71, 2016.
13. R.C. Aller, “Bioturbation and manganese cycling in hemipelagic sediments,” Phil. Trans. R. Soc. Lond, vol. 331, N 1616, pp. 51-68, 1990.
14. F.J. Millero, S. Sotolongo, M. Izaguirre, “The kinetics of oxidation of Fe(II) in seawater,” Geochim. Cosmochim. Acta, vol. 51, pp. 793-801, 1987.







