BISAC NAT010000 Ecology
BISAC NAT045050 Ecosystems & Habitats / Coastal Regions & Shorelines
BISAC NAT025000 Ecosystems & Habitats / Oceans & Seas
BISAC NAT045030 Ecosystems & Habitats / Polar Regions
BISAC SCI081000 Earth Sciences / Hydrology
BISAC SCI092000 Global Warming & Climate Change
BISAC SCI020000 Life Sciences / Ecology
BISAC SCI039000 Life Sciences / Marine Biology
BISAC SOC053000 Regional Studies
BISAC TEC060000 Marine & Naval
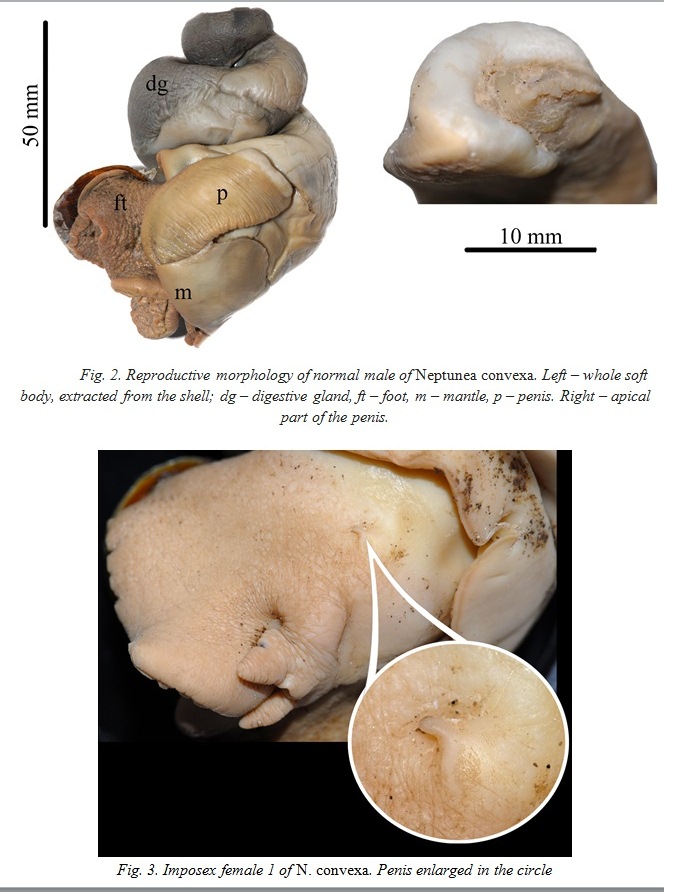
Owing to its worldwide use as an anti-fouling agent, tributyltin (TBT) is a common contaminant of marine ecosystems. Its wide distribution, high hydrophobicity and persistence have raised concern about bioaccumulation, potential biomagnifications in food webs, and adverse effects on the environment and human health. The most frequent and acute effect of TBT is found in gastropods, usually living in shallow waters, rarely at depths more than 100 m. This study reports about the first case of imposex in a deep water buccinid whelk Neptunea convexa collected at 1437 m in the Sea of Okhotsk. Among five collected specimens, the two were imposex females at the 1st stage of imposex development, while the rest three were males with normally developed penises. Most probably, TBT entered the whelk’s body by eaten benthic organisms, which feed on detritus with traces of TBT, but other reasons, such as heavy metal pollution, are also discussed.
imposex, pseudohermaphroditism, Neptunea, Gastropoda, TBT, tributyltin, heavy metals, pollution.
I. Introduction
Imposex or pseudohermaphroditism (appearing of male reproductive system characters in females of dioecious gastropods), was described in 70th years of the 20th century. At the moment, the most frequent and acute effect of TBT is found in gastropods: more than 150 mollusk species, predominantly belonging to Neogastropoda, have been reported to be affected [1]. It was shown that imposex has been caused by chemicals based on tributyltin (TBT), a common contaminant of marine ecosystems used as an anti-fouling agent. Its wide distribution, high hydrophobicity and persistence as well as capacity for bioaccumulation and potential biomagnification in food webs, produce adverse effects on the environment and human health. TBT causes endocrine disruption with androgen levels higher than normal which lead to masculinization in females. During last decades, imposex have been using as environmental pollution indicator. The majority of imposex cases were observed in sea port areas, in shallow waters not deeper than 100 m.
Our study reports about the first case of imposex in a deep water buccinid whelk Neptunea convexa.
II. Material and methods
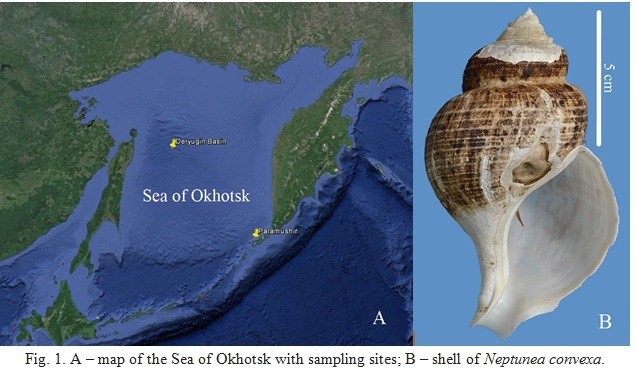
The material for the study was obtained in 61 cruise of R/V ”Academik M.A. Lavrentiev” (2013). The main objective of the cruise was investigation of the unique deep-water benthic ecosystems, formed in active fields on sites of cold seeps, with large amount of gas in their composition, usually methane. In particular, the zones of gas emanation with high methane concentration were examined in Deryugin Basin (the Sea of Okhotsk) at depths up to 1500 m (Fig. 1A). In point with coordinates 54°00.6′ N, 146°25.6′ E (depth 1431–1448 m, 2.3°С, salinity 34.4‰), by means of remotely operated underwater vehicle, 5 big specimens of Neptunea convexa (shell on Fig. 1B), and one specimen of Buccinum pemphigus were sampled. For comparison, we have studied buccinids, collected near North Kurile Islands (Paramushir Island, 50°30.9′ N 155°18.4′ E, 778 m) (Fig. 1A), with similar zones of gas emanation, i.e. N.insularus (1 specimen), Buccinum pemphigus (1 specimen), Ancistrolepis grammatus (1 specimen).
Fig. 1. A – map of the Sea of Okhotsk with sampling sites; B – shell of Neptunea convexa.
III. Results
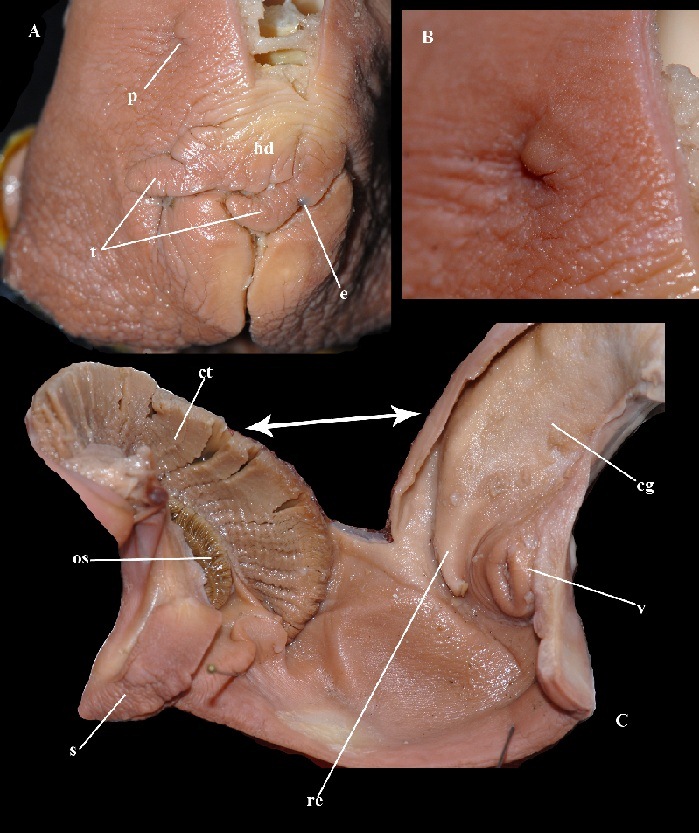
Fig. 4. Imposex female 2 of N. convexa. A – cephalopodium, e – eye, hd – head, p – penis, t – tentacle. B – penis enlarged. C – mantle, cg – capsule gland, ct – ctenidium, os – osphradium, re – rectum, s – siphon, v – vagina.
Table 1. Concentration of heavy metals in foot and digestive gland of buccinids from the Sea of Okhotsk, 770-1438 m.
|
Species |
Sampling site |
Foot, µg/g |
Digestive gland, µg/g |
||||||||||
|
Zn |
Cd |
Cu |
Ni |
Fe |
Ag |
Zn |
Cd |
Cu |
Ni |
Fe |
Ag |
||
|
Neptunea convexa, imposex female1 |
Deryugin Basin |
1763 |
53.6 |
100 |
2.1 |
210 |
3.4 |
4594 |
314 |
164 |
1.8 |
691 |
22.4 |
|
Neptunea convexa, imposex female2 |
Deryugin Basin |
618 |
24.2 |
58.7 |
5.8 |
72.2 |
2.3 |
11292 |
242 |
117 |
8.5 |
1170 |
9.3 |
|
Neptunea convexa, normal male1 |
Deryugin Basin |
1568 |
47.4 |
103 |
2.5 |
203 |
2.8 |
11558 |
564 |
155 |
1.3 |
828 |
7.7 |
|
Neptunea convexa, normal male2 |
Deryugin Basin |
1680 |
49.9 |
107 |
3.0 |
296 |
5.4 |
14848 |
373 |
340 |
2.4 |
2091 |
19.1 |
|
Alcohol |
Deryugin Basin |
448 |
3.3 |
366 |
0 |
0 |
0 |
919 |
8.4 |
415 |
0 |
0 |
0 |
|
Buccinum pemphigus |
Deryugin Basin |
591 |
24.8 |
97.4 |
11.7 |
66 |
2.3 |
6833 |
145 |
403 |
15.3 |
935 |
6.2 |
|
Buccinum pemphigus |
Paramushir
|
141 |
33.9 |
62.4 |
1.3 |
143 |
1.5 |
- |
- |
- |
- |
- |
- |
|
Neptunea insularis |
Paramushir
|
156 |
15.1 |
122 |
0.77 |
247 |
0.78 |
4392 |
267 |
253 |
1.7 |
1541 |
8.4 |
|
Ancistrole-pis grammatus |
Paramushir
|
198 |
10.8 |
42.0 |
1.9 |
903 |
0.36 |
- |
- |
- |
- |
- |
- |
|
Alcohol |
Paramushir |
- |
- |
- |
- |
- |
- |
202 |
0 |
0 |
0 |
34.1 |
11.6 |
IV. Discussion
Neptune whelks are typically dioecious, not having sex change and planktonic larvae during lifetime. Their feeding is still poorly studied, but there are evidences of predation on benthic invertebrates as well as scavenging [2, 3]. More recent determinations of the half-lives of TBT have shown them to be as high as 8 years in sediments and 4–17 years in the bivalves; the last are usually filtrators and are capable of bioaccumulation. These facts give reason to believe that TBT (which traces (Sn) we failed to detect via metal analysis probably due to inappropriate fixation of the material) entered the whelk’s body by eaten benthic organisms, which feed on detritus with traces of TBT. At the same time we cannot exclude other reasons of imposex, such as high concentration of other heavy metals (especially zinc and cadmium). Concentration of heavy metals in samples from Deryugin Basin was in majority of cases lower than in those from Paramushir. And average concentration of heavy metals, especially zinc and cadmium, was significantly higher than in other mollusks with high metal pollution, for example, those from Peter the Great Bay (the Sea of Japan) [4] and even hydrothermal vents [5]. There are evidences that lead pollution is connected with pseudohermaphroditism in Pugilina (Buccinoidea) [6], and copper induced imposex in experiments [7]. The imposex may be also a consequence of parasitic infection. Thus, larval stages of a trematode Stephanostomum baccatum were found in digestive gland of Buccinum undatum, and cadmium pollution was shown to increase susceptibility of snails Biomphalaria to infection with trematodes Schistosoma [8, 9].
IV. Acknowledgment
The study was supported by Russian Scientific Foundation grant (RSF project no. 14-17-00547).
1. P. Matthiessen, T. Reynoldson, Z. Billinghurst, D. Brassard, P. Cameron, G. Chandler, I. Davies, T. Horiguchi, D. Mount, J. Oehlmann, T. Pottinger, P. Sibley, H. Thompson, A. Vethaak, “Field assessment for endocrine disruption in invertebrates”. In: P. deFur, C. Crane, C. Ingersoll, L. Tattersfield, eds. Endocrine Disruption in Invertebrates: Endocrinology, Testing, and Assessment. Pensacola, FL: SETAC Press, pp. 199-270, 1999.
2. J. Pearce, G. Thorson, “The feeding and reproductive biology of the red whelk, Neptunea antiqua (L.) (Gastropoda, Prosobranchia),” Ophelia, vol. 4, no. 2, pp. 277-314, 1967.
3. J. Taylor, “The diet of Buccinum undatum and Neptunea antiqua (Gastropoda: Buccinidea),” Journal of Conchology, vol. 29, pp. 309-318, 1978.
4. V. M. Shulkin, V. Ya. Kavun, A. V. Tkalin, B. J. Presley, “The Effect of Metal Concentration in Bottom Sediments on the Accumulation of Metals by the Mytilids Crenomytilus grayanus and Modiolus kurilensis”, Russian Journal of Marine Biology, vol. 28, no. 1, pp. 43-51, 2002.
5. L. Demina., E. Shumilin, “Bioaccumulation of some trace elements in the biota of the hydrothermal fields of the Guaymas Basin (Gulf of California)”, Boletin de la Sociedad Geologica Mexicana, vol. 61, no. 1, pp. 31-45, 2009.
6. G. L. Sia Su, G. B. Ramos, E. C. B. Barcelon, R. M. C. Federo, M. L. L. Sia Su, K. Beltran-Benjamin, “Lead bioaccumulation and the imposex effect of Volema (Pugilina) cochlidium in Bacoor Bay, Philippines,” Journal of FisheriesSciences.com, vol. 9, no. 3, pp. 001-004, 2015.
7. D.J. Nias, C. McKillup, K. S. Edyvane, “Imposex in Lepsiella vinosa from Southern Australia,” Marine Pollution Bulletin, vol. 26, no. 7, pp. 380-384, 1993.
8. A.T. Sharaf El-Din, A.M. Mohamed, A.H. Mohamed, K.M. El-Hommossany, M.R. Habib, “Relationship between some heavy metals and Schistosoma mansoni infection rates in Biomphalaria alexandrina snails collected from different Egyptian localities,” World Applied Sciences Journal, vol. 11, no. 1, pp. 38-43, 2010.
9. A.T. Abd Allah, M.Q.S. Wanas, S.N. Thompson, “Effects of heavy metals on survival and growth of Biomphalaria glabrata Say (Gastropoda: Pulmonata) and interaction with schistosome infection,” J.Moll. Stud., vol. 63, pp. 79-86, 1997.