BISAC NAT010000 Ecology
BISAC NAT045050 Ecosystems & Habitats / Coastal Regions & Shorelines
BISAC NAT025000 Ecosystems & Habitats / Oceans & Seas
BISAC NAT045030 Ecosystems & Habitats / Polar Regions
BISAC SCI081000 Earth Sciences / Hydrology
BISAC SCI092000 Global Warming & Climate Change
BISAC SCI020000 Life Sciences / Ecology
BISAC SCI039000 Life Sciences / Marine Biology
BISAC SOC053000 Regional Studies
BISAC TEC060000 Marine & Naval
Fish yields of Ruditapes philippinarum have been decreased and the resources have not yet recovered. It needs to clarify food sources of R. philippinarum, and relationship between primary and secondary production of it. The purpose on this study is to reveal transfer efficiency from primary producers to R. philippinarum and food sources of R. philippinarum. The field investigation was carried out to quantify biomass of R. philippinarum and primary producers on intertidal sand flat at Zigozen beach in Hiroshima Bay, Japan. In particular, photosynthetic rates of primary producers such as Zostera marina, Ulva sp. and microphytobenthos were determined in laboratory experiments. The carbon and nitrogen stable isotope ratios for R. philippinarum and 8 potential food sources (microphytobenthos, MPOM etc) growing in the tidal flat were also measured. In summer 2015, the primary productions of Z. marina, Ulva sp. and microphytobenthos were estimated to be 70.4 kgC/day, 43.4 kgC/day and 2.2 kgC/day, respectively. Secondary production of R. philippinarum was 0.4 kgC/day. Contribution of microphytobenthos to R. philippinarum as food source was 56-76% on the basis of those carbon and nitrogen stable isotope ratios. Transfer efficiency from microphytobenthos to R. philippinarum was estimated to be 10-14%. It was suggested that microphytobenthos might sustain the high secondary production of R. philippinarum, though the primary production of microphytobenthos was about 1/10 compared to other algae.
Intertidal flat, Transfer efficiency, Primary and secondary production, Ruditapes philippinarum, Stable isotope.
I. INTRODUCTION
There are numerous of ecology study in R. philippinarum such as food sources [2] and function of purification [3] and larval recruitment [4] were conducted. Furthermore, it was reported that the cause of reduction in production of R. philippinarum were decline of primary production [5], hypoxia impact [6], deterioration in habitat [7], increment in predation [8]. It’s thought that a decrease of fish yields of R. philippinarum has been implicated as one of these important factors.
However, it needs for increment of fishery source to study and investigate, considering to understand ecology and environmental characteristic in habitat. Moreover, information on secondary production and their contribution to organic carbon pathways in various food webs is completely lacking. In this study, we estimated secondary production of R. philippinarum and provided quantitative estimates of the efficiency in channelling primary production to higher trophic levels. It needs for recovery of sources to clarify food sources of R. philippinarum, and relationship between primary and secondary production of it. The purpose on this study is to reveal transfer efficiency from primary producers to R. philippinarum and food sources of R. philippinarum.
Ⅱ. MATERIAL AND METHOD
Investigation area
Biomass and secondary production of R. philippinarum
Quadrat (50×50×10 cm) samples of R. philippinarum were collected to examine the abundance using 2 mm mesh sieve at 4 stations during the period from July 2014 to October 2015. Monthly investigation was carried out in white circle station in Fig.1. Investigaton of distribution of R. philippinarum was carried out in 27 black circle stations in Fig.1 including above station.
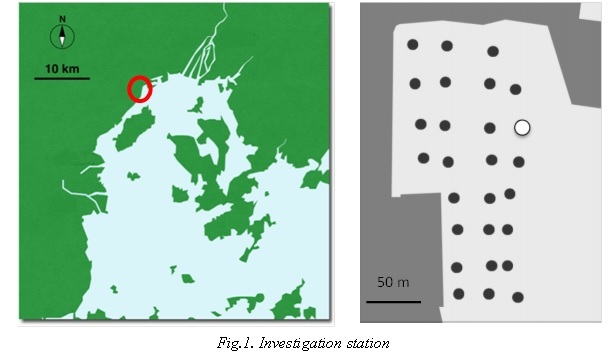
The shell length of R. philippinarum was measured using calipers. The Shell length- frequency data from R. philippinarum were analyzed. Cohort analysis was performed with the frequency distribution of shell length for secondary production.
Estimation of primary production of Microphytobenthos
Chl. a as microphytobenthos biomass in surface sediments which top 0-0.5 cm samples were collected by using an acrylic core tube (30 mm in diameter) and measured. Microphytobenthos were in situ surface sediments collect by exploiting their phototactic movement [9] during one day.
Primary production of microphytobenthos was measured by the 13C tracer method [10]. In the laboratory, microphtobenthos was suspended in filtered seawater and dispensed in polycarbonate bottle (500 mL).
After the addition of NaH13CO3 (about 10% of dissolved inorganic carbon), the samples were incubated for about 4 hours in a tank. The incubations were conducted at in situ temperature with running surface seawater under the corresponding light intensity, which was regulated by density filters. The light intensity inside the tank was about 200 µmol/m2/sec.
All the samples were filtered through precombusted 47 mm Whatman GF/F filter (450°C for 4 h), after incubation. The filters were treated with 1N HCl to remove inorganic carbon and washed by 3% NaCl. The filters were dried at 60oC and stored until isotope analysis.
Particle organic carbon concentration was analyzed by the elemental analyzer (Flash EA1112: Thermo Fisher Scientific), the 13C/12C isotope ratios in the filter samples were analyzed by Stable isotope ratio mass spectrometer (DELTAplus Advantage: Thermo Fisher Scientific).
Estimation of primary production of Macroalgae
Quadrat (30×30 cm) samples of macroalgae such as Zostera spp. and Ulva sp. were collected to examine the abundance at 3 each stations during the period from May 2015. In the laboratory, dry weight and carbon content of macroalgae were measured.
After filtering the seawater in the laboratory, the filtered seawater was sterilized using an autoclave (HIRAYAMA, HV-35LB). Zostera marina and Ulva sp. were cut a portion at a certain size to detect photosynthetic activity. The portion samples were incubated for about 2-4 hours incubator (NK system, LP-130P). The incubations were conducted at in situ temperature with running under light and dark condition. The light intensity was about 200 µmol/m2/sec. The dissolved oxygen concentration was measured before and after experiment (HACK, HQ40d). Primary production was calculated which photosynthesis quotient as 1.
Estimation of food resource of R. philippinarum
We collected R. philippinarum and potential 8 food sources such as microphytobrnthos (MPB), surface sediments (SOM), particulate organic matter of river origin (RPOM) and that of marine water (MPOM), Z. marina, Z. japonicaa, Ulva sp. and attached matter on Z. marina for measuring carbon and nitrogen stable isotope ratios (DELTAplus Advantage: Thermo Fisher Scientific).
SIAR 4.0 [12] was used to estimate the contribution of each potential primary producer source to R. philippinarum.
Ⅲ. RESULT AND DISCUSSION
Secondary production of R. philippinarum
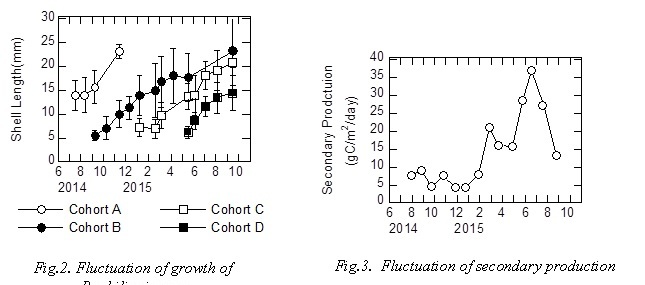
Figure 2 showed fluctuation of growth of R. philippinarum using cohort analysis. Two or three cohorts were detected during the sampling period. Density of R. philippinarum fluctuated 35-465 ind./m2 from August 2014 to October 2015. Figure 3 showed fluctuation of secondary production. It was estimated that secondary production was 4.3-37 mgC/m2/day. Secondary production was high value in early spring and summer.
Primary production of algae
Figure 4 shows fluctuation of photosynthetic activity and primary production. Photosynthetic activity of Ulva sp. was 30.0-76.5 mgC/dry g/day,photosynthetic activity of Z. marina was 14.3-40.6 mgC/dry g/day, photosynthetic activity of microphytobenthos was 4.48-22.1 mgC/mgChl.a/day. Though units of photosynthetic activity of microphytobenthos is different from the other macroalgae, photosynthetic activity fluctuation of the other macroalgae was greater than microphytobenthos. Photosynthetic activity of Ulva sp. was tendency higher than that of Z. marina. Photosynthetic activity of Z. marina did not cleary fluctuate.
Primary production of Ulva sp. was 3.29-21.1 gC/m2/day,primary production of Z. marina was 2.20-28.0 gC/m2/day, primary production of microphytobenthos was 0.074-0.240 gC/m2/day. For macroalgae, primary production of Ulva sp. increased in spring, primary production of Z. marina tended to have increased in summer. Primary production of microphytobenthos showed a maximum value in early summer, but primary production of microphytobenthos was smaller than that of the other macroalgae. Photosynthetic activity and primary production of microphytobenthos showed similar fluctuation.
.jpg)
Carbon and nitrogen stable isotope ratios
Figure 5 shows the carbon and nitrogen stable isotope ratios for R. philippinarum and 8 potential food sources. The δ13C and δ15N values of R. philippinarum were -18.7‰ to -15.1‰ and 10.4‰ to 14.4‰, respectively.
Figure 6 shows the contribution of 5 food resource for R. philippinarum. The plot in Fig 6 indicated range of 95% confidence interval. Contribution of microphytobenthos to R. philippinarum as food source was 56-76%, R. philippinarum mainly utilizes microphytobenthos as food source, contribution of MPOM (phytoplankton) and RPOM was less than 10%. Contribution of microphytobenthos to R. philippinarum was 35-64% in Hichirippu Lagoon bordering the Pacific Ocean in Hokkaido, Japan [13], it was high contribution of microphytobenthos to R. philippinarum as food source in this study.
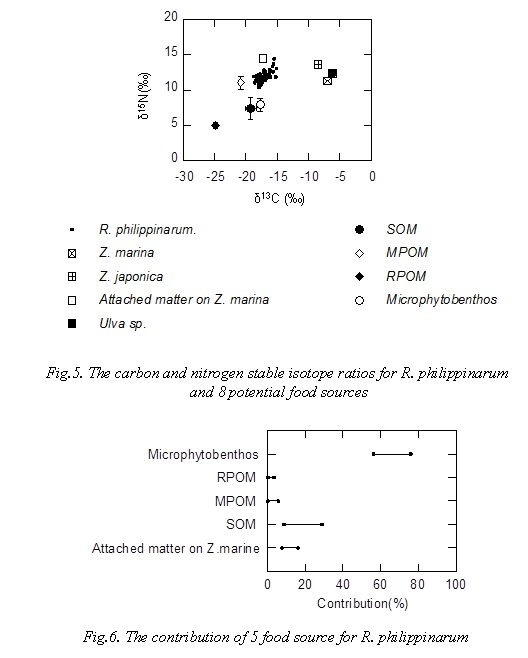
Transfer efficiency
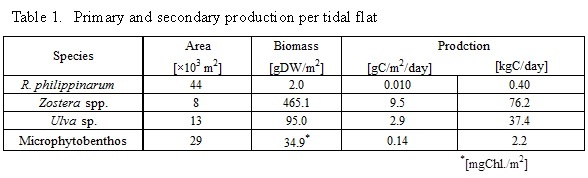
Table 1 shows primary and secondary production per tidal flat in summer 2015 from July to September. Area of R. philippinarum, Zostera spp., Ulva sp. and microphytobenthos were 44×103 m2, 8×103 m2, 13×103 m2 and 29×103 m2, respectively. The primary productions of Z. marina, Ulva sp. and microphytobenthos were estimated to be 70.4 kgC/day, 43.4 kgC/day and 2.2 kgC/day, respectively, considering of tidal flat area. Secondary production of R. philippinarum was 0.40 kgC/day. Contribution of microphytobenthos to R. philippinarum as food source was 56-76% on the basis of those carbon and nitrogen stable isotope ratios (Fig 6). Therefore, transfer efficiency from microphytobenthos to R. philippinarum was estimated to be 10-14%. It might efficiently transferred from primary production to secondary production in summer, because secondary production in summer was 5-6 times higher than that in winter, and highest through year (Fig.3).
Montani et al. [14] reported transfer efficiency from microphytobenthos to R. philippinarum was estimated to be 16% on Shin and Kasuga River tidal flat located in Seto Inland Sea, transfer efficiency in this study was substantially the same value. It thought that transfer efficiency in Montani et al. [14] was overestimated, since contribution of food resource has not been considered.
It was suggested that microphytobenthos might sustain the high secondary production of R. philippinarum, though the primary production of microphytobenthos was about 1/10 compared to other algae.
Ⅳ. CONCLUSION
- The primary productions of Z. marina, Ulva sp. and microphytobenthos were estimated to be 70.4 kgC/day, 43.4 kgC/day and 2.2 kgC/day, respectively.
- Contribution of microphytobenthos to R. philippinarum as food source was 56-76%, R. philippinarum mainly utilizes microphytobenthos as food source.
- Transfer efficiency from microphytobenthos to R. philippinarum was estimated to be 10-14%, microphytobenthos might sustain the high secondary production of R. philippinarum.
1. Ministry of Agriculture, Forestry and Fisheries, JAPAN., Annual Statistics of Fishery and Fish Culture, 2015.
2. Kasai, A., Horie, H., Sakamoto, W., Selection of food sources by Ruditapes philippinarum and Mactra veneriformis (Bivalva: Mollusca) determined from stable isotope analysis, Fisheries science, 70, 11-20, 2004.
3. Hiwatari, T., Kohata, K., Iijima, A., Nitrogen budget of the Bivalve Mactra veneriformis, and its significance in benthic-pelagic systems in the Sanbanse area of Tokyo Bay, Estuarine, Coastal and Shelf Science, 55, 299-308, 2002.
4. Ishii, R., Sekiguchi, H., Nakahara, Y., Jinnai, Y., Larval recruitment of the manila clam Ruditapes philippinarum in Ariake Sound, southern Japan, Fisheries science, 67, 579-591, 2001.
5. Hamaguchi, M., Relationship among primary production, marine environment and fisheries production of bivalves in the Seto Inland Sea, Bulletin of Fisheries Research Agency, 34, 33-47, 2011. (abstract in English)
6. Toba, M., Kosemura, T., Yamakawa, H., Sugiura, Y., Kobayashi, Y., Field and laboratory observations on the hypoxic impact on survival and distribution of short-necked clam Ruditapes philippinarum larvae in Tokyo Bay, central Japan, Plankton and Benthos Research, 3, 165-173, 2008.
7. Tsutsumi, H., Critical events in the Ariake Bay ecosystem: clam population collapse, red tides, and hypoxic bottom water, Plankton and Benthos Research, 1, 3-25, 2006.
8. Yamaguchi, A., Kawahara, I., Itoh, S., Occurrence, growth and food of longheaded eagle ray, Aetobatus flagellum, in Ariake Sound, Kyushu, Japan, Environmental Biology of Fishes, 74, 229-238, 2005.
9. Hama, T., Miyazaki, T., Ogawa, Y., Iwakuma, T., Takahashi, M., Otsuki, A., Ichimura, S., Measurement of photosynthetic production of a marine phytoplankton population using a stable 13C isotope, Marine Biology, 73, 31-36, 1983.
10. Couch, C.A., Carbon and nitrogen stable isotopes of meiobenthos and their food resource, Coastal and Shelf Science, 28, 433-441, 1989
11. Ichimi, K., Tada, K., Montani, S., Simple estimation of penetration rate of light in intertidal sediments, Journal of Oceanography, 64, 399-404, 2008.
12. Parnell, A.C., R. Inger, S. Bearhop, and A.L. Jackson., Source partitioning using stable isotopes: coping with too much variation. PlosOne, 5, e9672, 2010.
13. Komorita, T., Kajihara, R., Tsutsumi, H., Shibanuma, S., Yamada, T., Montani, S., Food sources for Ruditapes philippinarum in a coastal lagoon determined by mass balance and stable isotope approaches, PlosOne, 9, e86732, 2014.
14. Montani, S., Biological production of tidal flat: It's trophic dynamics, NIPPON SUISAN GAKKAISHI, 70(5), 796-797, 2004.







