с 25.12.2017 по настоящее время
с 26.12.2017 по настоящее время
с 26.12.2017 по настоящее время
BISAC NAT010000 Ecology
BISAC NAT045050 Ecosystems & Habitats / Coastal Regions & Shorelines
BISAC NAT025000 Ecosystems & Habitats / Oceans & Seas
BISAC NAT045030 Ecosystems & Habitats / Polar Regions
BISAC SCI081000 Earth Sciences / Hydrology
BISAC SCI092000 Global Warming & Climate Change
BISAC SCI020000 Life Sciences / Ecology
BISAC SCI039000 Life Sciences / Marine Biology
BISAC SOC053000 Regional Studies
BISAC TEC060000 Marine & Naval
Nonylphenol (NP) is the most abundant environmental estrogen listed as one of the priority hazardous substances in the Water Framework Directive (EC 2000) and the priority pollutant of Baltic Sea (HELCOM 2010). The present study aims to compare the effects of technical nonylphenol (tNP) on the cellulase, amylase and protease activity of the terrestrial fungal strains played a significant role in aquatic ecosystems due to their high adaptive capacity and a large range of functional activity. The study also attempts to understand the mechanisms behind the varying sensitivity of the terrestrial fungi to tNP. The fungal strains were isolated from the bottom sediments of the coastal area of the eastern part of the Gulf of Finland. The terrestrial fungi were identified based on their morphological characteristics and nucleotide sequence analysis of internal transcribed space region. One reason for significant differences in sensitivity to the toxicant studied among the fungi is the change in the fungal cell permeability, in particular in cell membrane permeability, induced by NP. Environmentally relevant concentrations of tNP cause significant changes in activity of hydrolytic enzymes in the terrestrial fungi Aspergillus tubingensis, Penicillium expansum, Penicillium glabrum, and Cadophora fastigiata involved in organic matter degradation in bottom sediments. There can be increasing or decreasing trend, depending on both the type of enzyme and the tNP concentration. The revealed changes may disrupt the destructive processes in bottom sediments, as well as succession and stability of microbial communities functioning in the aquatic environment. It was found that tNP contributes to the activation of proteolytic enzymes, considered as potential fungal virulence factors. This may lead to emergence fungal strains with enhanced virulence in aquatic microbiocenoses. The investigations of the physiological responses of terrestrial fungi under nonylphenol will be important for biochemical processes dynamics and their environmental consequences evaluation.
coastal area, nonylphenol, fungi, bottom sediments, hydrolytic enzymes activity
I. INTRODUCTION
During many years the
In recent decades, there has been increasing concern about environmental pollution with endocrine-disrupting chemicals (EDCs). Due to their widespread presence in the environment and toxic activity, EDCs have received increased attention in water quality management and health care. Among EDCs, nonylphenol holds a prominent place.
NP is the most abundant environmental estrogen listed as one of the priority hazardous substances in the Water Framework Directive (EC 2000) and the priority pollutant of Baltic Sea (HELCOM 2010). It is used for the production of nonylphenol polyethoxylates (NPEOs) which have been widely used as surfactants in industrial processes and households [4].
Nonylphenol enters the environment primarily through wastewater pathways. On entering aqueous environment, NP with its high hydrophobicity (log Kow 4.8–5.3) and low water solubility (5.43 mg/L at
Bottom sediments are sites of intense biogeochemical cycling regulated by microorganisms including terrestrial fungi. Fungi play a significant role in aquatic ecosystems due to their high adaptive capacity and a large range of functional activity. They show high efficiency in transforming organic substrates in aquatic ecosystems. Compared to other organisms, fungi are considered to be fairly resistant to toxicants. This is the reason why terrestrial fungi are often one of the dominant species in sediments contaminated with toxic chemicals [9].
At present, only a few studies have been conducted on NPʼs toxicity to fungi. Nonylphenol exerts toxic effects on the growth of filamentous fungi Neurospora crassa [10], Fusarium oxysporum and Fusarium solani [11], and Metarhizium robertsii [12]. Under growth suppression conditions, inhibition of fungal respiration and changes in fungal morphology were observed. In Neurospora crassa, Fusarium solani and Metarhizium robertsii under NP treatment cell shapes were abnormal and hyphal apical dominance was lost. These abnormalities were presumably due to disruption of the hyphal free cytosolic Ca2+ gradient, the H+ gradient, and the actin cytoskeleton of the apical cells [10]. In NP-treated Metarhizium robertsii samples, fungal hyphae exhibited ultrastructural changes at the cytoplasmic level, with major differences detected in vacuoles, mitochondria and cell walls [12].
Long-term exposure to low NP concentrations of 0.004 to 0.06 mg/L caused increased biomass production in the fungi Fusarium oxysporum and Fusarium solani. Moreover, a strong stimulation of spore production and germination was observed for Fusarium oxysporum [11].
However, until now, there have only been a few reports on the effects of NP on the physiological activity of filamentous fungi. Our previous studies have noted the influence of technical nonylphenol on cellulase and amylase activity of some terrestrial fungal strains of genera Aspergillus, Cladosporium, Exophiala, and Penicillium [13].
The present study aims to compare the effects of NP on the cellulase, amylase and protease activity of the terrestrial fungal strains isolated from the bottom sediments of the coastal area of the eastern part of the Gulf of Finland, which have different sensitivities to NP. The study also attempts to understand the mechanisms behind the varying sensitivity of the terrestrial fungi to NP.
- MATERIALS AND METHODS
Chemicals
Technical nonylphenol (CAS: 84852-15-3) was purchased from
The other chemicals were obtained from
Fungal strains and identification
The fungal strains Aspergillus tubingensis F11, Cadophora fastigiata F 17, Penicillium expansum F 44, and Penicillium glabrum F 41 used in this work were isolated from the bottom sediments of the coastal area of the eastern Gulf of Finland.
The fungal isolates were identified based on their morphological characteristics [14, 15] and a nucleotide sequence analysis of the internal transcribed space (ITS) region.
Genomic DNA was isolated using a reagent kit, an AxyPrep Multisource Genomic DNA Miniprep Kit (
Experimental set-up
The fungal cultures were grown in liquid media at
tNP dissolved in ethanol (125 mg/mL stock solution) was aseptically added to the culture media to reach the required concentration. The ethanol content, 0.04% v/v, was constant in all the variants. The control culture media were supplemented with the same amount of ethanol. The effect of ethanol (applied to dissolve tNP) on the growth of the fungi was found to be negligible (data not shown).
Permeability assays
For the analysis of the cell permeability, we used cultures of the fungi grown to stationary phase in a liquid Czapek medium with 2 % glucose.
The changes of the cell permeability of the terrestrial fungi exposed to tNP were monitored from the "loss" by the cells of metabolites exhibiting an absorption band in the ultraviolet (220-350 nm) [17]. A 200-mg weighed portion of mycelium was resuspended in 20 mL of distilled water and incubated for 1 h on a rotary shaker (230 rpm) at 30°С. The supernatant was analyzed with a Genesys 10 UV scanning spectrophotometer (
Hydrolytic enzymes assays
For the analysis of сellulolytic enzyme activity we used 7-day cultures of the fungi grown in a liquid Hutchinson medium with 1% sodium carboxymethylcellulose (Na-CMC). The enzymatic activity of cellulase was determined with the use of Na-CMC according to the procedures described by Li et al. [18]. The results were expressed in micrograms of glucose per microgram of protein.
The biomass production in these experiments was estimated from the resultant protein content determined by the method of Lowry et al. [19].
For the assay of proteolytic enzyme activity, the strains were grown in a liquid medium containing (in g/L) MgSO4 – 0.52; KCl – 0.52; KH2PO4 – 1.52; FeSO4·7H2O – 0.01; ZnSO4·7H2O – 0.01; glucose – 20.0; and albumin – 10.0 for 5 days. The extracellular proteolytic activity was determined according to the procedures described by Liu et al. [20]. The results were expressed as units per gram of dry weight biomass.
For the analysis of the amylase activity, we used 5-day cultures of the fungi grown in a liquid Czapek medium with 2% soluble starch at
Statistical analyses
All statistical analyses were performed with Statistica software (version 6; Statsoft). All of the data are presented as the mean ± SD of triplicates (n = 3). The data were tested with standard variance ANOVA, followed by Student’s t-test to determine significant differences. The differences were considered significant at P≤0.05
- RESULTS AND DISCUSSION
In this study we used the terrestrial fungal strains isolated from the bottom sediments of the coastal area of the eastern part of the Gulf of Finland.
The fungi were identified based both on the morphological characteristics according to the most common criteria [14, 15] and on analysis of the sequences of the ITS region of DNA.
For the investigation we selected terrestrial fungal strains that exhibited different sensitivities to NP (Table 1).
The strains investigated can be arranged in increasing order of their sensitivity to NP in the following sequence: Penicillium expansum F 44< Penicillium glabrum F 41<Aspergillus tubingensis F11< Cadophora fastigiata F 17. The ЕС50 value for Cadophora fastigiata F 17 strain, which is the most sensitive to tNP is 10-20 times lower than that for the strains from the genera Aspergillus and Penicillium.
Table 1. Toxicity parameters of tNP for terrestrial fungi
|
Fungal culture |
*ЕС50, mg/L |
*ЕС90, mg/L |
|
Aspergillus tubingensis F11 |
10.0 |
>100.0 |
|
Cadophora fastigiata F 17 |
1.0 |
7.0 |
|
Penicillium glabrum F 41 |
15.0 |
>100.0 |
|
Penicillium expansum F 44 |
20.0 |
>100.0 |
*ЕС50 and ЕС90 are the effective concentrations of 50 and 90% toxicant inhibition of fungal growth, respectively. The values of the toxicity parameters were calculated per 48 hours.
One reason for such significant differences in sensitivity to the toxicant studied among the fungi may be the change in the fungal cell permeability, in particular in cell membrane permeability, induced by NP.
The cytoplasmatic membrane is the primary target of negative impact of many chemical substances [22]. For all living cells, the ion transport through the cell membrane is essential for maintaining the ionic and osmotic homeostasis of the cell, as well as for information transfer, energy supply for cellular metabolism, substrate accumulation, and degradation products removal [23].
Permeability describes the ease with which ions can pass through a cell membrane to move substances into and out of the cell. Various toxicants have been reported to cause changes in permeability of fungal cell membranes [24, 25] in consequence of adaptation to the toxicant action. One factor that may be responsible for changes in cell membrane permeability is oxidation of membrane lipids [26]. Enhancement of membrane lipid peroxidation under NP-induced oxidative stress was observed in microalgae [27, 28].
Under tNP exposure, Cadophora fastigiata F 17 strain, the most sensitive to tNP, exhibited a 1.6-fold increase in the cellular permeability relative to the control (without tNP) (Table 2).
Table 2. Effect of nonylphenol on the cell permeability of the terrestrial fungi
|
Fungal culture |
tNP content, mg/L |
Permeability, % to control |
|
Aspergillus tubingensis F11 |
50.0 |
45±10 |
|
Cadophora fastigiata F 17 |
1.0 |
162±17 |
|
Penicillium expansum F 44 |
50.0 |
85±18 |
|
Penicillium glabrum F 41 |
50.0 |
91±14 |
Increased cellular permeability may facilitate the entry of toxicant into the cell, as well as the loss of vital metabolites. In tNP-resistant strains of the filamentous fungi the permeability of cell membranes either remained practically unchanged (Penicillium expansum F 44 and P. glabrum F 41) or significantly, by up to 55%, decreased (Aspergillus tubingensis F11) relative to the control variants (without tNP). These findings suggested that one possible mechanism behind high resistance of terrestrial fungi of genera Penicillium and Aspergillus is a decrease in the cell membrane permeability, which complicates the toxicant penetration into the cell.
Terrestrial fungi, which are an important component of aquatic ecosystems, including bottom sediments, possess a wide range of extracellular hydrolytic enzymes which enable them to actively degrade organic matter in the aquatic environment [9]. Therefore, the impact of tNP on the hydrolytic enzymes involved in fungal degradation of organic matter in water and bottom sediments is an issue that deserves special attention.
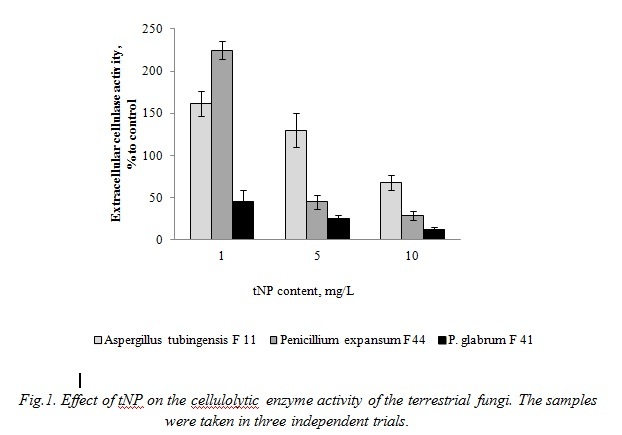
The cellulolytic enzymes performing biodegradation of cellulose, the most abundant biopolymer on Earth, occupy the central position in the organic carbon cycle. Among the terrestrial fungi isolated, Aspergillus tubingensis F11, Penicillium expansum F 44, and Penicillium glabrum F 41 strains exhibited cellulase activities. As shown by our previous study [29], the trend in the cellulase activity in the Penicillium expansum F 44 strain under the tNP influence depends on the tNP concentration. At low tNP concentrations (up to 1.0 mg/L) that do not significantly affect the Penicillium expansum F44 strain growth, the enzyme activity increased by 125% relative to the control (without tNP). An increase in tNP concentration in the medium to >1.0 mg/L resulted in both the culture growth inhibition and reduction in the cellulase activity, to 71% of the control value at tNP concentration of 10.0 mg/L. The cellulase activity of the Aspergillus tubingensis F11 strain was affected by tNP in a similar way (Fig. 1). By contrast to Penicillium expansum F 44 and Aspergillus tubingensis F11 strains, Penicillium glabrum F 41 exhibited a significant reduction in the cellulase activity both at low tNP concentrations that left the fungal growth practically unaffected (up to 5 mg/L) and at the growth inhibiting tNP concentrations (10.0 mg/L) (Fig.1).
Starch-hydrolyzing amylolytic enzymes were detected in all the terrestrial fungi investigated in this study. The tNP effect on the amylase activity of the terrestrial fungi was found to be species-nonspecific. Under tNP exposure, a decrease in amylase activity by 35 to 60%, depending on the species to which the strain belongs, was observed for all the strains investigated (Fig.2). It should be noted that the inhibitory effect of tNP on the amylase activity of the filamentous fungi was observed both at tNP concentrations that have no effect on the micromycete growth and at the growth inhibiting tNP concentrations.
.jpg)
Our previous study [13] has revealed similar effects from tNP treatments on the cellulase and amylase activities of other fungal strains of the genera Aspergillus, Penicillium, Сladosporium and Exophiala.
Along with cellulase and amylase activity, the activity of proteolytic enzymes as influenced by tNP exposure of the terrestrial fungi seemed to be an important research subject. This is due to the fact that not only these enzymes are known for their participation in protein breakdown in bottom sediments but also the secreted proteases have been intensively investigated as potential virulence factors of fungi [30].
Using the Aspergillus tubingensis F11 and Penicillium expansum F 44 strains as an example, we demonstrated that, under tNP exposure, the protease activity of the strains isolated increased 1.4-1.5 times (Fig.3).
.jpg)
In our previous studies [13, 29] we have shown that tNP can also increase the synthesis of other pathogenicity factors of fungi, pigments and polysaccharides.
Our data suggest that tNP has a potential to enhance fungal pathogenicity, which may lead to adverse environmental impacts, namely, to emergence of strains with enhanced virulence.
- CONCLUSIONS
Thus, terrestrial fungal species having different resistances to tNP were isolated from the bottom sediments of the coastal area of the eastern part of the Gulf of Finland. One reason for the differences in sensitivity to tNP among the fungi is presumably the disturbance of the cellular permeability. Environmentally relevant concentrations of tNP cause significant changes in activity of hydrolytic enzymes (cellulases, proteases and amylases) in the terrestrial fungi Aspergillus tubingensis, Penicillium expansum, Penicillium glabrum, and Cadophora fastigiata involved in organic matter degradation in bottom sediments. There can be increasing or decreasing trend, depending on both the type of enzyme and the tNP concentration. The revealed changes may disrupt the destructive processes in bottom sediments, as well as succession and stability of microbial communities functioning in the aquatic environment. It was also found that, along with enhancement of the synthesis of such fungal pathogenicity factors as pigments and polysaccharides, developed in fungi as adaptive mechanisms, tNP contributes to the activation of proteolytic enzymes, also considered as potential virulence factors. This may lead to emergence fungal strains with enhanced virulence in aquatic microbiocenoses. The investigations of the physiological responses of terrestrial fungi under nonylphenol will be important for biochemical processes dynamics and their environmental consequences evaluation.
1. A.E. Rybalko, N.K. Fedorova, “Bottom sediments of the Neva estuary and its contamination under influence of anthropogenic processes”, in: Ecosystem of the Neva Estuary: Biological Diversity and Ecological Problems (A.F. Alimov, S.M. Golubkov), Eds. KMK, St. Petersburg-Moscow, 2008, pp. 39-58 (in Russian).
2. H. Vallius, “Arsenic and heavy metal distribution in the bottom sediments of the Gulf of Finland through the last decade”, Baltica 25, 2012, pp. 23-32.
3. A.A. Eglit, N.V. Orlova, K.V. Ostrikov, A.V. Vlasov, V.M. Skvortsov, I.I. Murashko, et al., State of the Environment in Leningrad Region. Committee on Natural Resources of Leningrad Region, St. Petersburg, 2012, 320 pp. (in Russian).
4. A. Bergé, J. Gasperi, V. Rocher, L. Gras, A. Coursimault, R. Moilleron, “Phthalates and alkylphenols in industrial and domestic effluents: Case of Paris conurbation (France)”, Science of the Total Environment, 2014, pp. 26-35. doihttps://doi.org/10.1016/j.scitotenv.2014.04.081.
5. Y. Kim, G.V. Korshin, A.B. Velichenko, “Comparative study of electrochemical degradation and ozonation of nonylphenol”, Water Res, 2005, vol. 39, pp. 2527-2534.
6. D.Y. Shang, R.W. Macdonald, M.G. Ikonomou, “Persistence of nonylphenol ethoxylate surfactants and their primary degradation products in sediments from near a municipal outfall in the strait of Georgia, British Columbia, Canada”, Environ. Sci. Technol., 1999, vol. 33, pp.1366-1372.
7. A. Soares, B. Guieysse, B. Jefferson, E. Cartmell, J.N. Lester, “Nonylphenol in the environment: a critical review on occurrence, fate, toxicity and treatment in wastewaters”, Environ. Int., 2008, vol. 34, pp. 1033-1049.
8. R. Vazquez-Duhalt, F. Marquez-Rocha, E. Ponce, A. F. Licea, M.T.Viana, “Nonylphenol, an integrated vision of a pollutant. Scientific Review”, Applied Ecology and Environmental Research, 2006, vol. 4 (1), pp. 1-25.
9. V.A.Terekhova, The fungi in ecological assessment of the water and terrestrial ecosystems, Мoscow, Nauka, 2007, 215p. (in Russian).
10. A.J. Karley, S.I. Powell, J.M. Davie, “Effect of nonylphenol on growth of Neurospora crassa and Candida albicans”, Applied and Environmental Microbiology, 1997, vol. 63 (4), pp. 1312-1317.
11. A. Kollmann, A. Brault, I. Touton, J. Dubroca, V. Chaplain, C. Mougin, “Effect of nonylphenol surfactants on fungi following the application of sewage sludge on agricultural soils”, Journal of Environmental Quality, 2003, vol. 32 (4), pp. 1269-1276.
12. S. Rozalska, S.Glinska, J. Dlugonsky, “Metarhizium robertsii morphological flexibility during nonylphenol removal”, International Biodeterioration and Biodegradation, 2014, 95. pp. 285-293.
13. I.L. Kuzikova, E.A. Tileva, T.B. Zaytseva, N.G. Medvedeva, “Effect of nonylphenol on terrigenous fungi of the coastal zone of the eastern Gulf of Finland”, Mikologiya i fitopatologiya, . 2015, vol. 49 (4), pp. 249- 256. (in Russian).
14. K.H Domsch, W. Gams and T.H. Anderson, Compendium of soil fungi: Volume 1, 1980, Academic Press, London, p. 859.
15. R.A. Samson, and E.S.Van Reenen-Hoekstra, Introduction to food-borne fungi, 3rd ed. 1988, Baarn, p. 295.
16. T.J. White, T. Bruns, S. Lee, J. Taylor, 1990. “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics”, in: PCR Protocols: a guide to methods and applications, (M.A. Innis, D.H. Gelfand, J.J. Sninsky, T.J. White, eds), Academic Press, New York, USA, pp. 315-322.
17. B.A. Fenderson, E.M. Eddy, S.I. Hakomori, “Glycoconjugate expression during embryogenesis and its biological significance”, BioEssays, 1990, vol. 12 (4). pp. 173 -179.
18. F. Li, X. Zhu, N. Li, P. Zhang, S. Zhang, X. Zhao, et al., “Screening of Lignocellulose-Degrading Superior Mushroom Strains and Determination of Their CMCase and Laccase Activity”, Scientific World Journal, 2014, Article ID 763108, 6. doi: https://doi.org/10.1155/2014/763108.
19. O.H. Lowry, N.J. Rosebrough, A.L. Farr, R.J. Randall, “Protein measurement with the Folin phenol reagent”, J. Biol. Chem., 1951, vol. 193 (1), pp. 265-275.
20. F. Liu, W. Li, D. Ridgway, T. Gu, and M. Moo-Young, “Inhibition of extracellular protease secretion by Aspergillus niger using cell immobilization”, Biotechnology Letters, 1998, vol. 20, (6), pp. 539-542.
21. D.K. Sandhu, K.S. Vilkhu, and S.K. Soni, “Production of α-amylase by Saccharomyces fibuligera”, J. Ferm. Technol, 1987, vol. 65(4), pp. 387-394. doihttps://doi.org/10.1016/0385-6380(87)90134-8.
22. G. McDonnell and A. D. Russell, “Antiseptics and Disinfectants: Activity, Action, and Resistance”, Clin Microbiol Rev, 1999, vol. 12 (1), pp. 147-179.
23. B. Alberts, A. Johnson, J. Lewis, M. Raff, K. Roberts, P. Walter, Molecular Biology of the Cell, Garland Science, New York, USA, 4th edition, 2002.
24. N. Medvedeva, Yu. Polyak, I. Kuzikova, O. Orlova, G. Zharikov, “The effect of mustard gas on the biological activity of soil”, Environmental Research, 2008, vol.106, pp. 289-295.
25. C. Yang, C. Hamel, V. Vujanovic, and Y. Gan, “Fungicide: Modes of Action and Possible Impact on Nontarget Microorganisms”, International Scholarly Research Network, 2011, vol. 2011, Article ID 130289, 8 p. doihttps://doi.org/10.5402/2011/130289.
26. X.X. Tang, T.J. Jan, Y.Q. Li, “Damage effect of monocrotophos on Platymonas sp. I. Active oxygen in Platymonas sp. cells”, Chinese Journal of Applied Ecology, 1998, vol. 9 (6), pp. 627-630.
27. N. Medvedeva, T. Zaytseva, I. Kuzikova, “Cellular responses and bioremoval of nonylphenol by the cyanobacterium Planktothrix agardhii 1113”, Journal of Marine Systems, 2016, in press.
28. H. Qian, X. Pan, S. Shi, S.Yu, H. Jiang, Z. Lin, Z. Fu, “Effect of nonylphenol on response of physiology and photosynthesis-related gene transcription of Chlorella vulgaris”, Environ. Monit. Assess, 2011, vol.182, pp. 61- 69.
29. I. Kuzikova, V. Safronova, T. Zaytseva, N. Medvedeva, “Fate and effects of nonylphenol in the filamentous fungus Penicillium expansum isolated from the bottom sediments of the Gulf of Finland”, Journal of Marine Systems, 2016, in press.
30. M. Monod, A. Fatih, L. Jaton-Ogey, S. Paris and J.P. Latge, “The secreted proteases of pathogenic species of Aspergillus and their possible role in virulence”, Can. J. Bot., 1995, vol. 73, pp. 1081-1086.







