с 26.12.2017 по настоящее время
с 25.12.2017 по настоящее время Россия
с 25.12.2017 по настоящее время Россия
BISAC NAT010000 Ecology
BISAC NAT045050 Ecosystems & Habitats / Coastal Regions & Shorelines
BISAC NAT025000 Ecosystems & Habitats / Oceans & Seas
BISAC NAT045030 Ecosystems & Habitats / Polar Regions
BISAC SCI081000 Earth Sciences / Hydrology
BISAC SCI092000 Global Warming & Climate Change
BISAC SCI020000 Life Sciences / Ecology
BISAC SCI039000 Life Sciences / Marine Biology
BISAC SOC053000 Regional Studies
BISAC TEC060000 Marine & Naval
The factors that provoke fish suffocation in an estuary, namely: natural (small river runoff, high air and water temperature, water stratification) and anthropogenic (regulation of river, etc.) were marked. Taking into account these factors the calculations were carried out and the possible areas of the Dnieper-Bug estuary, where fish kill of different scale and genesis is found out were identified.
water quality, fish suffocation, modeling, marine estuary.
I. Introduction
A large fish dying is a result of various anthropogenic impacts. It requires integrated exploration and detailed research. This phenomenon has the largest importance when the extreme situation has occurred on the water basin. In other words, a large fish dying is the threat for the ecosystem in whole.
To find out periodicity, areas, and possible reasons for an origin of a large fish dying and to work out the ways of avoiding such accidents in near future it needs to gather information, namely: water environment and weather conditions, incidents on various economic venues and etc.
The analysis of available information about suffocation phenomena in the
The aim is to analyze the extreme deterioration of water quality during suffocation phenomena in the sea mouth of the Dnieper and the
II. Statement of problem
The mouth of the Dnieper and the South Bug rivers is located in the northwest
In summer the water level in the
The central part of the Dnieper-Bug estuary is not used by fish fauna for spawning. Close to the channel, areas can not serve as a full-fledged feeding area, and fishing areas. And fishing and feeding areas are confined to the shallow water. On the shallow water the water is usually mixed up decently and is saturated with oxygen. However, in summertime a lack of oxygen is often observed that is explained by the number of factors. The slowing of river velocity is of great importance during windless weather in hot summer. At temperature variations with depth enlargement the static zone appears. The photosynthetic processes take place on the surface where algae saturate water with oxygen. Also the algae are missing in the deep lightless layers. The fish activity declines while being in water with low oxygen concentration. The fish resistance to diseases and unfavorable environmental factors considerably reduces [1].
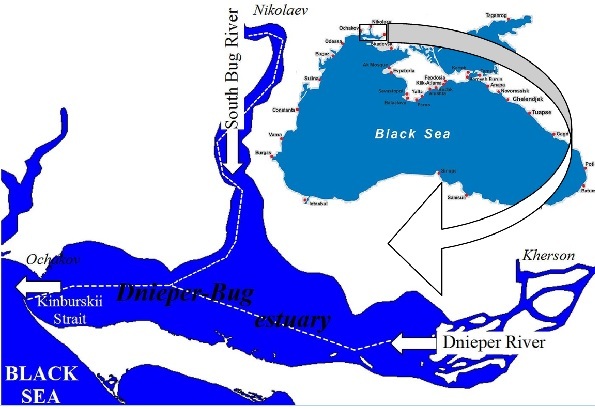
Fig.1. The map of the Dnieper-Bug estuary showing the location of the navigation channel (white dashed line).
Also there are some differences in the dying fish species. If earlier there were bullhead, roach, kilka, silverside, crucian carp, flounder, but in some years mainly bullhead died (fish aged 1 – 4 years). This could be explained by biological peculiarities of this fish. That includes: small size, and as a result physical impossibility to get out of suffocation area.
A high amount of oxygen in water is used by excessive developing and further dying of blue-green algae in summer. During blooming this algae products oxygen in day-time and absorbs it at night, especially in early morning. By these entire facts oxygen gap appears in nonmoving ponds. And this is one of the reasons why fish die.
The blue-green algae significantly develop in so-called “blooming spots”. They usually appear in windless bays; although it depends on the year conditions, “blooming spots” may appear in open part of the water area. Also when biomass and its covered territory become larger the parts of “blooming spots” may be separated from the main body. And these parts begin to migrate on the water area because of the discharged current and wave influence.
Anthropogenic impact, even if it is not the main reason of the large fish dying, may increase its scope. Polluted environment reduces fish resistance to various external factors (temperature change, mineralization, gas regime, etc.).
To the factors given below we should add the intrabasin process of water quality forming, particularly, the polluting matter deposit in bottom sediments and their entering into water again when the certain conditions of water area were changed (pH, dissolved oxygen, etc.).
Among abiotic parameters of water ecosystem the bottom sediments hold a special place, because they are capable to concentrate different chemical substances (such as heavy metals, nature and anthropogenic organic matters, the products of their destruction, radionuclide, etc.). Depending on the water area conditions the bottom sediments may serve as pollution accumulator (self-purification) or as a source of pollution income to water (secondary pollution) [2].
The negative factors of ecological state in natural water area are dredging, containment on underwater soil dumps, sand extraction and other activities in the water. The increasing turbidity area is formed where the exchange of suspension and pollutants is happened. The feeding fish migration and spawning habitat are constantly decreasing. This leads to extreme decreasing of fish population and has bad influents on reproduction of the main native industrial fish species.
Frequently all negative above-listed facts below affect together, so that they strengthen each other. The examples of suffocation phenomena with description of hydrodynamic parameters are listed below.
In [3] the suffocation phenomena were explored in the Nikolaev Region in 2010 when seven events were registered from 4 to 23 August. The biggest activity of fish suffocation phenomenon was registered in the Black Sea on 5 August near the Koblevo village, on 6 August in the
Near 1 mil 280 thousand fish specimens have died in the estuary on 10 August 2010.
As it was explained in the ecological administration the content of chlorides, sulphates and phosphates in the water was extremely higher than the maximum permissible concentration (MPC) for the commercial fishing water areas. The content of chlorine was 2532.36 – 4459.34 mg/dm3 (MPC = 300 mg/dm3); of sulphates 326.81 – 619.05 mg/dm3 (MPC = 100 mg/dm3); of phosphates 0.85 – 1.69 mg/dm3 (MPC 0.17 mg/dm3). According to official data from the State Ecological Inspection in Kherson region a high content of hydrogen sulphide from 2.22 to 7.03 mg/dm3 was found in the Dnieper-Bug estuary.
III. Methods of investigation
The 3-D unsteady hydrothermodynamic Model for Estuarine and Coastal Circulation Assessment (МЕССА) [4] was used for the set of experiments about dynamic analyses of thermohaline water structure and current patterns in the low-water period and under different wind directions. The 1-D biogeochemical unit of the water eutrophication model is the system of interdependent differential equations, which describe biogeochemical cycles of biogenic elements, production and destruction of organic matter, oxygen dynamics at a local point of the water environment.
The rest state was taken as initial conditions. A homogeneous mass was taken in the horizontal plane as the thermohaline water structure at the initial time. The water environment was approximated into a horizontal grid 91 ´ 97 nodes with pitch at 600 m where the model was accommodated. The calculation was made in condition of low-water level when the discharge was 440 m3 per sec for the Dnieper River and 46 m3 per sec for the South Bug River.
IV. Results of research
The salt water distribution in the estuary is provided on Fig.2. The wind and wave water mixing is impeded in windless and low-water level conditions. Fresh water gets into the surface layer of estuary with river runoff. But in the bottom water layer we can find high salinity water of marine origin (10 – 14 ‰), because the salinity water flows on the navigation channel.
.jpg)
Fig.2. The graphical output showing the results of the hydrological model: salinity on the surface (a) and the bottom (b) layers under no wind condition.
In the Dnieper-Bug estuary the fish suffocation phenomena are the most seen in the navigation channels, leading to Nikolaev and Kherson. Here hypoxia is developing in the navigation channel and this leads to the forming of the stable infesting hydrogen area where aquatic organisms die yearly. The fish suffocation phenomena is mostly seen in August (27% cases), in July (19% cases), it is less seen from October to March (0 – 8% cases) [1]. In the Kinburskii Strait the suffocation phenomenon is a rare fact, because there is a high level of flowing water and hydrodynamic activity.
.jpg)
The surface and bottom current patterns in windless period are given on Fig.3. The water circulation pattern shows that in the windless and low-water period the surface flow is separated on two parts. One flow is strained along the northeast shore of the Bug estuary. Second flow is strained along the south shore of the estuary. The bottom flows are seen in the navigation channel. There are either gradient or compensational currents. In the bottom area flows are strained along the navigation channel in the whole water column. Than when the flow get the middle it bifurcates and its part get into the Bug estuary.
The river runoff dominates in the upper water layer, especially in the shallow areas of the west regions. The intensity of the Black Sea influence decreases in the other way. The water bloom is evolving because in summer the Dnieper River discharge is 500 m3/s or less. In such condition the closed areas of increased sediment load appear in the central estuary part. They usually are in the position with the place of blue-green algae bloom.
The suffocation phenomena are less seen in the west area of the Dnieper estuary where the self-purification capacity of water is higher. Because more clear water gets through the Kinburskii Strait and the wind and wave activity is higher than in other estuary areas where the disposal of waist takes place.
The runoff flows speed reduces in several hours when the wind stops. The smaller river runoff and bigger water mass homogeneity in the estuary, the deeper the wind flows extend.
The modeling patterns of the surface and bottom currents in the surging wind 5 m/s is shown on Fig.4. The runoff flows are reduced under the influence of the surging wind especially in the surface water level because the wind currents are spread through all water body. It causes the water outset. The skewed level appears (Fig.3, b). Because of these the flows in the surface water level set in to the side of level rising. That is why the contradictions appear due to gravity in the bottom water level such as compensation and gradient flows. In the north shallow water area the drift flows prevail and in the navigational channel there are compensational currents.
.jpg)
It is known that wind-induced surges in the estuary have the influence on its gas regime, in particular on oxygen content in the water. When the water reservoir discharges water volume more than 1500 m3/s, and the winds have north directions, the estuary are desalinated and oxygen is distributed as well as in a river water: in the vegetation period in the surface layer the oxygen content reaches a maximum (20 – 23 mg/l), in the coastal areas even 30 mg/l; in the bottom layer its content is slightly lower. Under long set-up or calm water at the bottom (mainly in the navigation channel) the anaerobic zones appear, where there is no oxygen and hydrogen sulphide is presented.
The sea water surges reduce the intensity of photosynthesis process in the surface layers in the pelagial and littoral. Absolute and relative content of oxygen in water decreases with chloride growth (a chlorides increment 200 – 500 mg/l means an oxygen loss of
2 – 3 mg/l). In this regard, the lowest oxygen content is observed in the west region and in the Bug estuary, the largest one is in the east. It can explain not only the hydrological regime of each area, but also the degree of human impact.
Upper layer is saturated with oxygen and the generated organic matter enters in bottom layers and sediments. The velocity of oxygen process is bigger by increasing temperature that leads to decrease of oxygen concentration in natural layer. That may cause hypoxia which provokes hydrogen sulfide and suffocation phenomena. The annual dynamic of bottom water layer is shown on Fig.5 whish was calculated using 1-D version MECCA model. Oxygen maximum was in winter months (9 – 10 g/m3) when the water temperature is low. The concentration decreases in spring and from the middle April it is lower than optimal standard that is established for commercial fishing water bodies (MPC = 6 gO2/m3) [1]. Hypoxia is from July to October (0.5 – 1.5 gO2/m3). Then the oxygen consumption decreases with lowering of temperature. This explains why oxygen value is higher in autumn and winter
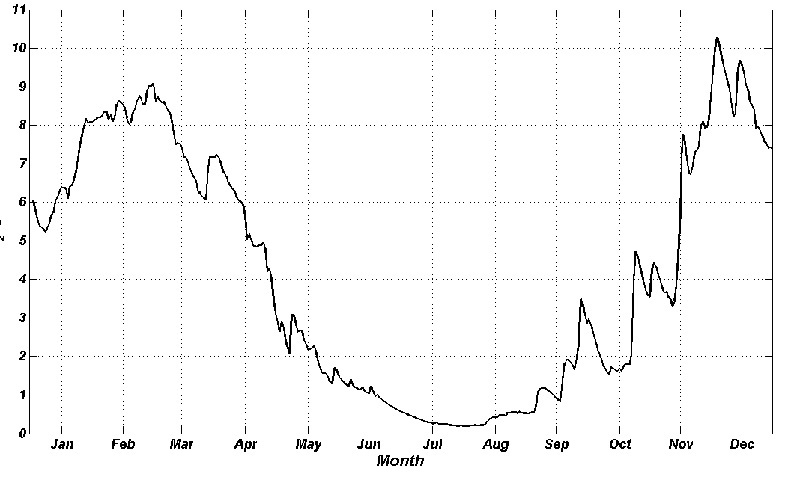
Fig.5. Distribution of oxygen on the bottom layer of the Dnieper-Bug estuary throughout the year, obtained from MECCA.
It is also known that in summer when there is excessive development and future die-off blue-green algae the large amount of oxygen dissolved in water is consumed. To study the phytoplankton dynamic we constructed the annual dynamics of phytoplankton biomass on the surface of the Dnieper-Bug estuary using 1-D version model MECCA.
The annual cycle of mean monthly value about chlorophyll concentration in this area is needed. This information may be get from the space scanner data. For example, SeaWiFS and MODIS-Aqua (http://oceancolor.gsfc.nasa.gov) which measure the ocean color change and the spaceborne scanners are used very often to determine optic characteristics of sea water and biochemical measurements. However the standard algorithm of space scanner data processing in SeaWiFS and MODIS-Aqua often gives incorrect estimation of concentration. For example, in [5] the overestimate chlorophyll concentration which was taken from the satellite data for the Barents Sea, the Black Sea and the Caspian Sea is shown incorrectly. This value was larger in several times than measuring data. That is why the required chlorophyll concentration in the Dnieper-Bug estuary was taken from [6] where was used the author’s regional algorithm to calculation bio-optical parameters of the Black Sea according to satellite ocean color data.
The concentration of phytoplankton biomass is minimal in winter and in early spring (0.075 – 0.125 gC/m3). The maximum of blooming is in summer and in early autumn (0.2 – 0.25 gC/m3) (Fig.6). In spring the flood is going on the Dnieper and the South Bug Rivers which increases the input of biogenic and organic matter to the Dnieper-Bug estuary. Also the length of day and photosynthetic process increase. As a result the biomass of phytoplankton grows.

Fig.6. Distribution of biomass of phytoplankton on the surface layer of the Dnieper-Bug estuary throughout the year, obtained from MECCA.
V. Conclusions
The cause of fish suffocation phenomena is the combination of several negative ecological factors both natural and anthropogenic origin [7].
Natural facts are high temperature, lack of wind mixing in water ecosystem that reduces secondary pollution.
Anthropogenic facts are lack of water exchange before fish dying; storage of organic in water both easy and difficult oxidized; high concentrations of heavy metals and pesticides in water and bottom sediments that are capable to weaken the protective mechanisms of fish and other aquatics and make them vulnerable to dramatic changes in environment; low oxygen concentration in high temperature; organic oxidation; hydrological conditions.
The suffocation phenomena damage is following: economical damage to commercial fishing and other services such as fish utilization service. The ecological damage is an increase of biodiversity and food chain destroy. The restore of estuary requires near 2 or 3 years.
To solve the suffocation phenomena the following ways are: preventive measures for overgrowing pretension with sea grass (it is recommended to cut sea grass during blooming); to exclude some fish species in order to optimize quality and quantity composition; to force the control for polluting sources in water during summer; to form interdepartmental commission for complex research on the fish suffocation phenomena stopping and remediation; to hold explanatory conversation within locals about the rules of conduct on water and prevention ways of the suffocation phenomena.
To develop protective measures, strategy of ecological accidents is necessary to organize complex observation of these events, to restore hydro-biological monitoring which was not carried out during last 20 years, to develop methods for forecast of water quality deterioration.
The operational model of ecosystem in the mouth of the Dnieper and the South Bug could be an analyze suffocation phenomena and forecast instrument.
1. V.N. Zhukinsky, L.A. Zhuravleva, A.I. Ivanov et al., Dnieper-Bug estuarine system. Kiev: Naukova Dumka, 1989. 374 p.
2. P.N. Linnik, “Influence of various factors on the metal desorption from bottom sediments in the conditions of experimental modeling” // Hydrobiological Journal. 2006. 42, № 3. pp. 97-114.
3. O.O. Shumіlova, and G.G. Trokhimenko, “Study of mass fish suffocation phenomenon of in the Nikolaev Region in August, 2010” // Electronic Journal. 2010. № 5.
4. V.A. Ivanov, and Yu.S. Tuchkovenko, Applied mathematical water-quality modeling of shelf marine ecosystems. Sevastopol: ECOSY-Hydrophysics, 2008. 311 p.
5. O.V. Kopelevich, V.I. Burenkov, S.V Ershova, et al., “Application of SeaWiFS data for studying variability of bio-optical characteristics in the Barents, Black and Caspian Seas” // Deep-Sea Research II. 2004. vol. 51. pp.1063-1091.
6. O.V. Kopelevich, V.I. Burenkov, S.V. Sheberstov, et al., Bio-optical characteristics of the seas of Russia from data of the SeaWiFS satellite ocean color scanner. CD-ROM. Moscow. SIO RAS. 2005.
7. R.Yu. Minkovskaya, Local environmental disaster in the Dnieper-Bug mouth area // Ecological safety of coastal and shelf zones and complex usage of shelf resources. Sevastopol: ECOSY-Hydrophysics, 2010. 23. pp.166-170.







