BISAC NAT010000 Ecology
BISAC NAT045050 Ecosystems & Habitats / Coastal Regions & Shorelines
BISAC NAT025000 Ecosystems & Habitats / Oceans & Seas
BISAC NAT045030 Ecosystems & Habitats / Polar Regions
BISAC SCI081000 Earth Sciences / Hydrology
BISAC SCI092000 Global Warming & Climate Change
BISAC SCI020000 Life Sciences / Ecology
BISAC SCI039000 Life Sciences / Marine Biology
BISAC SOC053000 Regional Studies
BISAC TEC060000 Marine & Naval
“The Healthy Plan of Enclosed Coastal Environments” was a project implemented from 2011 to 2013 to design an effective action plan for restoring a healthy environment in Mitsu Bay, Hiroshima, Japan. After collecting and analyzing natural and social background information, two controversial issues associated with the bay’s ecosystem were identified: a decrease in oyster production that may be due to oligotrophication of the bay, and deterioration of sediment quality caused by oyster culture. Four mitigation approaches were proposed by the committee and their effectiveness was evaluated using numerical calculations. An application of hot air-dried oyster shells (HACOS) to the sediment was considered the most effective measure, because the remediation of sediment quality by adsorbing hydrogen sulfide may increase benthos biomass and fisheries production. Improved conditions were estimated to continue for 10 years. The application of HACOS is easy for fishermen, and is very cost effective because it is a by-product of extensive oyster culturing in the bay. Thus, the approach is also considered advantageous for establishing a recycling-orientated community. The effective action plan for sustainable fisheries and restoration of the environment outlined in this study is proposed as a leading model of design and implementation.
action plan, coastal environment, ecosystem, modelling, sustainability, oyster shell
I. Background
“The Healthy Plan of Enclosed Coastal Environments” was a project conducted from 2011 to 2013 by the Ministry of Environment, Japan to design an effective action plan for the unhealthy environment in Mitsu Bay, Higashi-Hiroshima (Fig. 1). Mitsu Bay covers 25 km2 and averages 10 m deep, with an open mouth to the Seto Inland Sea. Although Mitsu Bay is not geographically closed (water retention time in the bay has been calculated as ca. 2.5 days [1]), the environmental conditions of the bay were considered to be “not healthy and need thorough investigation” during preliminary checks [2].
In addition to the decrease in population in the catchment area from ca. 16,000 at its peak in 1947 to ca. 12,000 at present, a sewage treatment plant (STP) started operations in 2007. These factors may have caused diminished primary production and increased transparency of water in the bay [3]. Fisheries statistics showed decreased Nori (Porphyra yezoensis) and Manila clam (Ruditapes philippinarum) production in the late 1970s and 1980s, respectively, and both are at almost zero production today. From these trends, the bay could be in an oligotrophic condition as observed in other areas of the Seto Inland Sea [4, 5].
However, large-scale oyster culture is conducted in the bay, and production increased in the 1960s, with a peak in fresh meat production of ca. 2,000 tons recorded in 1965, followed by a reduction to half that level at present. Despite this reduction, oyster culture remains the main fishery. It may be the cause of organic matter loading to the seafloor, even though Mitsu Bay is not geographically closed.
Monthly data on water quality have been published and made available on the internet [6]. However, there were previously no sediment quality data available for the bay. As a preliminary investigation, we measured acid volatile sulfide (AVS) of the sediment, as AVS provides an indicator of anaerobic conditions. Sediment samples collected on four occasions (November 2011, January, June and August 2012) at five stations (Stations 2–6) in the northern part of the bay where the sediment quality is supposed to be deteriorated (Fig. 1) showed that AVS was high at Station 5, with values higher than the Japanese standard (0.2 mg/g dry sediment) [7]. However, there was no indication of dissolved oxygen depletion in the bottom water either by vertical profile measurements from the sea surface to 0.5 m above the seafloor at 12 stations (Stns. 1-12) or during 15 days of monitoring conducted at four mooring stations (Stns. 1, 5, 6 and 7) in August 2012. Thus, partial deterioration of sediment quality may have been caused by massive feces loading by the oyster culture, despite no deterioration in water quality.
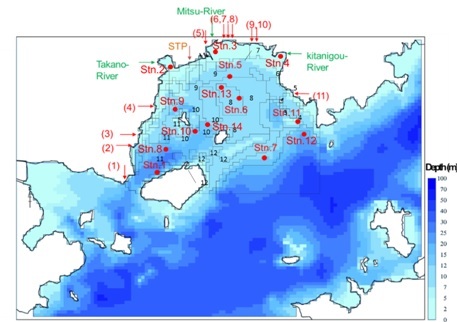
|
Fig. 1. Map showing the geographical features of Mitsu Bay. Arrows indicate the sites of river mouths of major three rivers, sewage treatment plant (STP) and major industries (1-11) which were taken into considerations in the model as input sources of N, P and organic matter. Blocks with number indicate the area with similar characteristics for which the material calculation was made. |
In the present study, our aim was to form a local committee and propose suitable mitigation measures for Mitsu Bay through observations, data analyses and evaluation of possible approaches with the aid of computer simulations.
II. Materials and Methods
Based on the concept of the Law for the Promotion of Nature Restoration established in 2002 by the Ministry of the Environment [8], we organized a local committee composed of representatives from different fields to reflect the various opinions of stakeholders. The committee included the chiefs of the fisheries and environmental divisions of Higashi-Hiroshima City, the secretaries and chiefs of the fisheries and environmental divisions of Hiroshima Prefecture, the presidents of two fisheries unions, and five academic experts. The first author of this study, Tamiji Yamamoto, was selected as the chairperson of the committee.
Following a review of the literature and hearing from fishermen and citizens living in the Mitsu Bay area, the committee identified two major issues: a decrease in oyster production possibly due to the oligotrophic state of the seawater, and deteriorated sediment quality, particularly below the oyster culture rafts because of excess oyster feces. The committee selected four approaches to address these issues and improve material circulation in the bay. We selected (i) relaxation of effluent from the STP to enhance primary production, (ii) reduction of cultured oyster numbers to reduce feces sedimentation, (iii) an application of fertilizer as a measure to improve the oligotrophic conditions, and (iv) an application of materials to improve sediment quality.
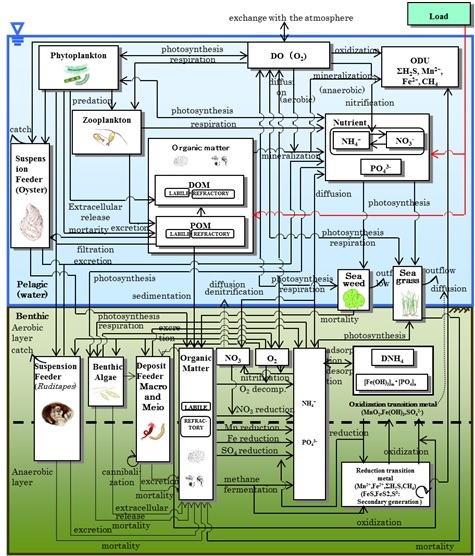
|
Fig. 2. Schematic depiction illustrating framework of the pelagic-benthic coupling model constructed in the present study. Compartments are illustrated with rectangles and processes are shown with arrows. |
To evaluate the effectiveness of these four approaches, we applied a pelagic-benthic coupling ecosystem model combined with an advection-diffusion physical circulation model [9]. In the physical circulation model, a nesting procedure was implemented: a 100 × 100 m grid was applied to the area inside Mitsu Bay, and a 300 m or 900 m grid was used for the area outside of the bay. The water column was divided into 13 vertical layers. Data pertaining to the freshwater inflow from rivers and the water temperature and salinity in the bay for 2001–2009 were obtained from the Water Quality Survey of Public Water Area [6]. For climatic conditions, data such as solar radiation and precipitation were obtained from 13 land stations located in the calculation area [10]. Additional observations were also carried out at four stations in Mitsu Bay in winter and summer 2012, along with the data collection from the moored current meters, as mentioned in I. Background. Sediment traps were also deployed at Stations 5, 13 and 14 to measure the downward particulate flux. The calculation for the material cycles were carried out by blocks, not by pixel as shown in Fig. 1, which corresponded with areas with similar characteristics, e.g., oyster culture area; areas covered with thin, thick or no eelgrass.
As illustrated in Fig. 2, nine variables were included in the pelagic part of the ecosystem model; ammonia, nitrate+nitrite, phosphate, dissolved organic matter, particulate organic matter, phytoplankton, zooplankton, filter feeder mainly oyster and DO. Eight variables were incorporated into the benthos component of the model; ammonia, nitrate+nitrite, phosphate in the interstitial water, total organic matter, benthic microalgae, detritus feeder, filter feeder mainly manila clam, and DO. Physiological processes of oysters and material cycles in oyster rafts were the same as described in our previous paper [11], and were based on the existing literature [12]. Eelgrass and seaweeds were also included between the pelagic and benthic components. Equations and parameters used for the material cycles in eelgrass beds and tidal flats were the same as those previously described [9, 13]. Additionally, dissolved iron, manganese, and H2S were incorporated as the oxygen demanding unit in the model to calculate the oxidation-reduction processes that occur in the sediment and sediment water interface. Equations and parameters used for the oxidation-reduction processes were the same as those described in our previous study [14], and were also referenced from the existing literature [15].
For the first approach mentioned above, we modeled an increase in concentrations of dissolved inorganic nitrogen (DIN) and dissolved inorganic phosphorus (DIP) in the effluent water from the STP from the present averages of 3.35 mg N/L and 1.91 mg P/L to 20.0 mg N/L and 2.0 mg P/L, respectively. These increased concentrations are the maximum allowable levels for maintaining healthy water quality in Japanese coastal seas according to the Water Pollution Control Act [16]. We increased the dissolved inorganic forms because those are the well-treated forms from the advanced tertiary treatment system at the STP. If untreated wastewater is released as forms of TN and TP, including particulate matter, it can become problematic for oyster cultures in terms of coliform and noroviruses [17]. For the second approach, we changed the raft numbers in the model to 0.5–1.5 times the present number to investigate how the ecosystem would respond. Modeling of the third approach was carried out for three levels of nutrient additions according to different applications of fertilizer: Case 1: 324 kg N/day and 65 kg P/day, Case 2: 97 kg N/day and 19.5 kg P/day, and Case 3: 32 kg N/day and 6.5 kg P/day. Assuming DIN and DIP additions to Block 7 (Fig. 1) for 2 months (01 October to 30 November) during the oyster growing season, calculations were made for water quality, sediment quality and oyster growth.
For the fourth approach, we selected hot air-dried crushed oyster shells (HACOS) from several potential means of remediating sediment. The reasons for this include that oyster shells naturally occur in the sea so applying them in a processed state is justifiable. HACOS is easily obtained at low cost because the Hiroshima area has the highest level of oyster production in Japan, and industries exist whereby oyster shells are processed by heating and crushing for use as additives to soil to improve quality. Additionally, HACOS has been scientifically proven in its effectiveness for the reduction of hydrogen sulfide (H2S) [18, 19]. In the present study, we carried out field experiments to confirm the current body of published scientific evidence. On 23 July, 2013, we selected a 5 × 5 m experimental plot (Exp) below the oyster culture raft at Station 5 in Block 7 where the H2S concentration was at a record high in the bay. Divers plowed one tons of HACOS, which had approximately 1/3 of the sediment volume that was being mixed with, into the plot to a depth of 10 cm. Another two plots of the same areal size were selected; for the control plot with no treatment (Cont), and for the plowed plot without the addition of HACOS (Plow). Three sediment samples were collected, each from 3 plots (triplicate sampling) in July (before treatment), August (1 month later) and October (3 months later) 2013. The AVS content in the sediments and H2S concentrations in the interstitial water were measured for each sample. In August, oxygen consumption rates and dissolved nitrogen and phosphorus release rates were also measured for intact sediment cores collected by divers from Exp and Cont by incubating them in a temperature controlled incubator (EYELA MTI-201, Tokyo). Duplicate measurements were made for each plot.
For the fourth approach, the numerical model calculation was carried out assuming that HACOS was applied to Blocks 7, 8, and 9 (Fig. 1) where sediment quality was the worst.
III. Results and Discussion
Relaxation of effluent from the STP
Relaxing the effluent load from the STP to the maximum DIN and DIP levels showed no significant change in TN concentrations and phytoplankton chlorophyll a (Chl a) concentrations in Mitsu Bay waters [20]. In our estimation, the loads from the STP effluent in the first year were 1.0 kg N/day and 0.6 kg P/day in winter and 0.5 kg N/day and 0.9 kg P/day in summer, which accounted for 1.8–5.8% N and 24–25.7% P of the total loads from all possible sources. This suggests that the amount of STP effluent initially processed at the plant is not very large; one quarter of the population residing in the catchment area. Furthermore, the water retention time in the bay of ca. 2.5 days suggests physical diffusion may dilute the released effluent in a short time. Thus, we concluded that this measure may not be effective if used in combination with other measures.
Reduction of cultured oyster numbers
Increasing cultured oyster numbers to 1.5 times of the existing numbers in Mitsu Bay slightly decreased Chl a concentrations and increased H2S concentrations in the sediment interstitial water by 12%. Conversely, by reducing the cultured oyster numbers to half of the existing numbers, Chl a concentrations increased slightly in the northern area of the bay and H2S concentrations in the sediment interstitial water decreased by 14%. The decrease in H2S concentrations in the sediment may lead the benthic ecosystem toward healthier conditions.
Although the total biomass yield of cultured oysters did not differ among cases, individual weight increased with decreasing cultured oyster numbers (Fig. 3). This suggests that the phytoplankton concentrations may not be sufficient for oyster growth under the current conditions. Although we did not estimate the increase in fishermen’s incomes with the increase in individual oyster weight, a trade-off is expected between the increase in unit price and the decrease in the produced total oyster numbers. Reducing cultured oyster numbers requires a sensitive approach for fishermen, because a decrease in the cultured oyster numbers can directly affect their income. Therefore, if fishermen want to maintain the existing high total oyster production without reducing oyster numbers, an addition of fertilizer might be required to enhance phytoplankton biomass for the oyster feed. This should be investigated further with additional calculations to assess economic implications.
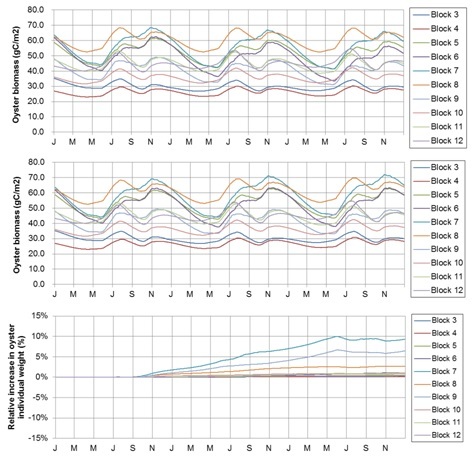
Fig. 3. Seasonal change in the total oyster biomass by increasing to 1.5 times (upper) and decreasing to half of the present number (middle). Lower panel shows the individual oyster weight in each block at the number lowered to half.
Application of fertilizer
The addition of fertilizer was effective in Case 1 but was not obvious in Cases 2 and 3, whereby low amounts of nutrient were added. The highest load (Case 1: 324 kg N/day and 65 kg P/day) was much higher than the estimated riverine load (ca. 20 kg N/day and 1.4 kg P/day on average) and effluent from the STP (0.5–1.5 kg N/day and 1.0 kg P/day). Although fertilizer was added to Block 7 (Fig. 1), the distribution of the added nutrients shifted right to Block 6. As a result, Chl a concentration increased in Block 6, but not in Block 7, because of the influence of residual flow. This suggests that the addition of fertilizer in liquid form would be challenging because of the difficulty in predicting which site would exhibit the effect. Instead, we have developed a solid form of the fertilizer, which can be applied by hanging below the raft and may be effective for several months [21]. Application of HACOS to the sediment
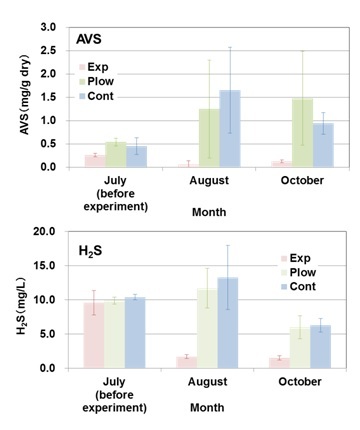
|
Fig. 4. Changes in acid volatile sulfide (AVS) content of the sediments and hydrogen sulfide concentrations in the interstitial water of the sediments before (July, 2013) and after (August and October, 2013) applying the hot air-dried crushed oyster shells (HACOS) to the bottom sediments of reduced condition. To the experimental plot (Exp), HACOS was applied approximately 1/3 of the sediment volume that was being mixed with, into a depth of 10 cm, 5 m x 5 m. Another two plots of the same areal size were set; for the control plot with no treatment (Cont), and for the plowed plot without the addition of HACOS (Plow). Experiments were carried out at Station 5 in Block 7 where the H2S concentration was at a record high in the bay. |
AVS content was almost the same at the three plots in their initial condition in July with ca. 0.5 mg/g dry sediment, and suppressed close to zero in Exp in August and October (Fig. 4a).
Conversely, AVS at Plow and Cont increased to ca. 1–1.5 mg/g dry as the season progressed. H2S concentrations in the interstitial water were also suppressed from 10 mg S/L of the initial value to less than 2 mg S/L at Exp, whereas the values at Plow and Cont showed a general seasonal change, increasing in summer and decreasing in autumn with no apparent suppression (Fig. 4b). Although the oxygen consumption rate for the water overlying the sediment was not significantly different among the 3 plots, nutrient release rates were significantly suppressed in Exp compared with Cont, particularly for dissolved phosphorus (Table 1). Benthos significantly increased by individual number in October at Exp compared with Cont, although species numbers did not significantly differ (39 in Exp and 40 in Cont). Polychaetes were the dominant benthos both in Exp and Cont, but the dominant species differed. Schistomeringos rudolphi dominated in Exp and Scoletoma longifolia in Cont, with the latter reported as an indicator species of polluted sediment [22].
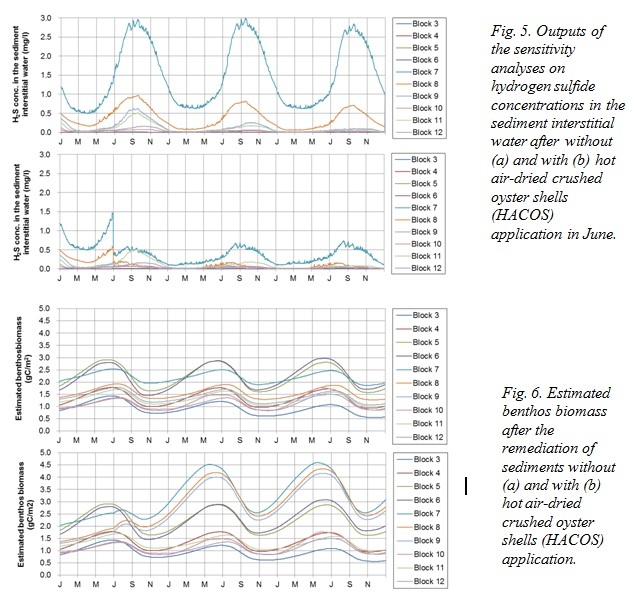
The model simulation showed that the H2S concentration in the interstitial water was suppressed, particularly in Block 7 by 70% by the end of the 3-year simulation (Fig. 5). The cumulative amount of adsorbed H2S 2.5 years after the application of HACOS was estimated to be 3.15 mg S/g shell, which equates to 1.17 mg S/g shell/yr. As maximum adsorption capacity is reported to be 12 mg S/g shell, the effect of HACOS is considered to last 10 years. Remediation of sediment quality may lead to an increase of benthic animals (Fig. 6), and therefore increased fish production because of the increased fish food availability. Our calculations estimated an increase of 31.2% in fish biomass, from 852 kg C at present to 1,118 kg C in the entire bay following HACOS application.

IV. Summary and recommendations
We conducted a 3-year project organized and supported by the Japanese Ministry of the Environment to design an effective action plan for sustaining local resources and managing the coastal environment of a local bay, Mitsu Bay in Hiroshima. Following a review of the literature and hearing from fishermen and citizens living in the area, we identified that the bay had a number of controversial issues. These included a decrease in oyster production possibly due to the oligotrophic state of the seawater, and deteriorated sediment quality, particularly below the oyster culture rafts because of excess oyster feces. To address these issues, four approaches were proposed by the project committee. The effectiveness of each approach was evaluated using a pelagic-benthic coupling model, and additional field experiments were also conducted for some of the measures. Among the proposed approaches, the application of HACOS was most effective in restoring the health of the ecosystem. The HACOS application was not only effective in remediating sediment quality but could also be valuable for enhancing fishery production through an increase of benthic organisms available for fish to feed on. Using oyster shells to restore the Mitsu Bay ecosystem is also important in terms of promoting a recycling-orientated community, because oyster shells are a by-product of the local oyster fisheries in this area.
We recommend our process as a template when designing and implementing an effective action plan, while acknowledging that our study was based on a single case study and that circumstances will be different according to the local area. Our process focuses on finding the issues of the target area, collecting background information on natural and social environments, using experts from a committee to propose hypotheses, selecting management approaches, carrying out additional experiments and/or field surveys where required, and modeling the scenarios to understand the effectiveness of each measure, then designing an action plan that may be most suitable for the local ecosystem and community in terms of their sustainability. However, flexibility in the form of adaptive management is required for this kind of procedure if the proposed approach is not as effective as planned and expected at the initial stage. We hope this methodology will be adopted as a general style for other local areas with similar problems.
V. Acknowledgment
We thank the Ministry of the Environment, Japan for financial support of this research project.
1. Sanyo Techno Marine, Inc., Report on the Project of Planning and Investigations on Healthy Material Cycles of the Seas - Mitsu Bay-, 2013. (in Japanese with English abstract)
2. The Ocean Policy Research Institute, Report on Physical Check of the Seas 2008. 312 pp. (in Japanese)
3. Sanyo Techno Marine, Inc., Report on the Project of Planning and Investigations on Healthy Material Cycles of the Seas - Mitsu Bay-, 2012. (in Japanese with English abstract)
4. T. Yamamoto, “The Seto Inland Sea-Eutrophic or oligotrophic?” Mar. Poll. Bull., vol. 47, pp. 37-42, 2003.
5. T. Yamamoto, and T. Hanazato, Oligotrophication of Coastal Seas and Lakes. Chijin Shokan, Tokyo: 195 pp., 2015. (in Japanese)
6. Ministry of the Environment, http://www.env.go.jp/water/suiiki/ (viewed April 22, 2016).
7. Japan Fisheries Resource Conservation Association, Standard for Fisheries Water. 68 pp., 1995. (in Japanese)
8. Ministry of the Environment, http://www.env.go.jp/nature/saisei/law-saisei/ (viewed April 22, 2016).
9. K. Hata and K. Nakata, “Evaluation of eelgrass bed nitrogen cycle using an ecosystem model,” Environ. Model. Software, pp. 491-502, 1998.
10. Japan Meteorological Agency, http://www.jma.go.jp/jma/index.html (viewed April 22, 2016).
11. J. Kittiwanich, T. Yamamoto, O. Kawaguchi and T. Hashimoto, “Analyses of phosphorus and nitrogen cyclings in the estuarine ecosystem of Hiroshima Bay by a pelagic and benthic coupled model”, Est. Coast. Shelf Sci., vol. 75, pp. 189-204, 2007.
12. M. Kobayashi, E. E. Hofmann, E. N. Powell, J. M. Klinck, and K. Kusaka, “A population dynamics model for the Japanese oyster, Crassostrea gigas,” Aquaculture, vol. 49, pp. 285-321, 1997.
13. K. Hata, K. Nakata and T. Suzuki, “The nitrogen cycle in tidal flats and eelgrass beds of Ise Bay,” J. Mar. Syst., pp. 237-253, 2004.
14. H. Yamamoto, T. Yamamoto, S. Asaoka, and Y. Mito, “Numerical evaluation of the use of granulated coal ash to reduce an oxygen-deficient water mass,” Mar. Poll. Bull., (20160401, accepted).
15. H. Fossing, P. Berg, B. Thandrup, S. Rysgaard, H. M. Sørensen and K. Nielsen, A Model Set-up for an Oxygen and Nutrient Flux Model for Aarhus Bay (Denmark). NERI Technical Report, pp. 5-61, 2004.
16. Ministry of the Environment, http://law.e-gov.go.jp/htmldata/S45/S45HO138.html (viewed May 9, 2016).
17. Hiroshima Prefecture, https://www.pref.hiroshima.lg.jp/soshiki/58/1291003977219.html (viewed May 9, 2016) (in Japanese, partially in English).
18. S. Asaoka, T. Yamamoto, S. Kondo, and S. Hayakawa, “Removal of hydrogen sulfide using crushed oyster shell from pore water to remediate organically enriched coastal marine sediments,” Biores. Technol., vol. 100, pp. 4127-4132, 2009.
19. T. Yamamoto, S. Kondo, K. H. Kim, S. Asaoka, H. Yamamoto, M. Tokuoka, and T. Hibino, “Remediation of muddy tidal flat sediments using hot air-dried crushed oyster shells,” Mar. Poll. Bull., vol. 64, pp. 2428-2434, 2012.
20. Sanyo Techno Marine, Inc., Report on the Project of Planning and Investigations on Healthy Material Cycles of the Seas - Mitsu Bay-, 2014. (in Japanese with English abstract)
21. T. Yamamoto, S. Takahashi, T. Kiyota, Y. Kawajiri, and K. Takeda, “Development of iron supplying fertilizer for restoration of coastal marine ecosystems,” Fish. Eng., vol. 51, pp. 105-115, 2014. (in Japanese with English abstract)
22. Hiroshima Environment and Health Association, Research Report on Ecological Remediation of Sediment Quality using Microalgae. 28 pp with supplementary tables and figures, 2004. (in Japanese)







