BISAC NAT010000 Ecology
BISAC NAT045050 Ecosystems & Habitats / Coastal Regions & Shorelines
BISAC NAT025000 Ecosystems & Habitats / Oceans & Seas
BISAC NAT045030 Ecosystems & Habitats / Polar Regions
BISAC SCI081000 Earth Sciences / Hydrology
BISAC SCI092000 Global Warming & Climate Change
BISAC SCI020000 Life Sciences / Ecology
BISAC SCI039000 Life Sciences / Marine Biology
BISAC SOC053000 Regional Studies
BISAC TEC060000 Marine & Naval
We studied for the first time luminescence characteristics of the some micromycetes, isolated from the bottom sediments of the Black sea from the 27 m depth. Luminescence parameters were registered at laboratory complex “Svet” using mechanical and chemical stimulations. Fungi cultures of genera Acremonium, Aspergillus, Penicillium were isolated on ChDA medium which served as control. Culture of Penicillium commune gave no light emission with any kind of stimulation. Culture of Acremonium sp. has shown luminescence in the blue – green field of spectrum. Using chemical stimulation by fresh water we registered signals with luminescence energy (to 3.24 ± 0.11)•108 quantum•cm2 and duration up to 4.42 s, which 3 times exceeded analogous magnitudes in a group, stimulated by sea water (p < 0.05). Under chemical stimulation by ethyl alcohol fungi culture luminescence was not observed. Culture of Aspergillus fumigatus possessed the most expressed properties of luminescence. Stimulation by fresh water culture emission with energy of (3.35 ± 0.11)•108 quantum•cm2 and duration up to 4.96 s. Action of ethyl alcohol to culture also stimulated signals, but intensity of light emission was 3–4 times lower than under mechanical stimulation. For sure the given studies will permit not only to evaluate contribution of marine fungi into general bioluminescence of the sea, but as well to determine places of accumulation of opportunistic species in the sea.
microscopic fungi (micromycetes), luminescence characteristics, chemical stimulation.
- Introduction
Despite the fact that bioluminescence is known for many vegetable and animal systematic groups for several thousands of years, contribution of marine fungi into the seas and oceans luminescence has not been studied [3, 8, 15]. Moreover practically nothing is known about marine mycobiota luminescence. Two alternative ideas are under discussion: first, land tree fungi luminescence is provided by the classic enzyme – substrate system of luciferase – luciferine and, to the contrary, an idea that fungi luminescence causes oxidation of the organic substrates without specialized enzyme participation. Fungi represent by themselves the third kingdom of the living nature, evolutionary distanced from the plants and animals that is why learning of their luminescence mechanism is fundamental interest. Biochemistry of the fungi is quite peculiar and gives us opportunity to expect that their bioluminescent system can appear to be principally another [1]. By the present time 1500 microscopic fungi species have been isolated from the marine environment, among them about 500 are higher obligate marine fungi known only for marine or water habitats. Representatives of Ascomycota section dominate in their species composition – 97% (including their anamorph forms, according to old classification group of Anamorphic Fungi (17%) [5].
Actuality of the studies of micromycetes different groups living in the sea is proved by their role in the ecosystem. Some widely spread in environment saprophyte fungi cause secondary mycoses and allergic diseases in men and hydrobionts. Such fungi are called opportunistic, representatives of genera Aspergillus (A. flavus, A. fumigatus, A. terreus), Fusarium (F. moniliforme, F. oxysporum), Alternaria, Cladosporium, Penicillium belong to them [6]. In the ecosystems of moderate altitudes with high anthropogenic load they clearly observe a tendency of the potentially pathogenic fungi accumulation with high rate of growth. It has been stated that often the same fungi species are resistant to several anthropogenic factors [10]. Just these fungi species inhabit different substrates and they are revealed by luminescent methods.
Eutrophication and pollution of the sea facilitate micromycetes development: on one hand they participate in decomposition of the organic and chemical substances, on the other hand they parasite on hydrobionts immunity of which is weaken by the unfavorable environmental conditions [14, 16]. At present time known hundreds of substances (toxins, growth stimulators, organic acids, hormones, vitamins etc), which are synthesized and accumulated in the microscopic fungi cells (micromycetes) and in the culture medium (liquid). As a rule they are specific for separate species and are called secondary metabolites. Obligate marine fungi are mostly connected with cellulose – containing substrates, thus resembling luminescent land fungi. Considering all above mentioned we aimed our work on determination of luminescent ability and the biophysical parameters of bioluminescence studies in separate species of the micromycetes and their complexes, isolated from the marine habitats.
II. Materials and methods
Beginning from 30-ties of the last century in medicine, veterinary and food industry fluorescent diagnostics use for revealing microscopic fungi and their metabolites on the skin and hair coverings of the animals and man, books, plastics, meat, fish, vegetables, corns, milk products, wine. Diagnostics is based on ability of fungi and substances they synthesize to produce light under the long-wave ultraviolet rays influence. The studies of the fungi metabolite products and their ability to produce light are conducted in mycelium cells and cultural medium [7, 9, 12]. To create ultraviolet radiation they use practically different devices (Wood lamp, hand lamp Vista UV handle etc) [18, 19, 21, 22, 23].
Using such methods in the Institute of marine biology, National Academy of Science of
.jpg)
To compare characteristics of the facultative and marine fungi luminescence we studied also cultural liquid of the fungi complexes isolated from the Black sea scallop valves, crab carapax and fragments of wood gathered at the coast (Table 1). Fungi complexes were chosen according to the greatest fluorescence under ultraviolet lamp EVT – 01 irradiation (light flow 780 lm, wave length 360 nm) (Fig. 2). Substrate and culture of micromycetes were separated from the cultural liquid by filtration through the paper filter “white band” (specific weight –
Obligate and facultative fungi species from Ascomycota section, represented by the anamorph and teleomorph stages made part of the mycocomplexes. Anamorh is an organ of sexless or vegetative reproduction of fungi. Teleomorph is a form of sex spore – containing in fungi, its purpose – genes recombination and fungus protection under unfavorable conditions or wintering. Anamorph and teleomorph have different names and morphologically and cariologically clearly differ one from another. Some fungi species are known only at one of reproductive stages.
Table 1. Species composition of the micromycetes complexes
|
|
Substrate, medium |
||||||||||
|
|
SG |
К |
W |
W |
W |
W |
W |
W |
W |
W |
ChDA |
|
Micromycetes species |
Age of a complex, months |
||||||||||
|
|
22 |
25 |
24 |
33 |
12 |
7 |
10 |
18 |
7 |
15 |
1.2 |
|
Anamorph stages |
|||||||||||
|
Alternaria alternata (Fr.) Keissl, 1912 |
+ |
– |
– |
– |
– |
– |
– |
+ |
– |
– |
+ |
|
Dendryphiopsis sp. |
|
– |
– |
– |
– |
– |
– |
+ |
+ |
– |
– |
|
*Dictyosporium pelagicum (Linder) G. C. Hughes, 1963 |
|
– |
– |
+ |
– |
– |
– |
– |
– |
– |
– |
|
*Cirrenalia macrocephala (Kohlm) Meyers, R. T. Moore, 1960 |
– |
– |
– |
+ |
+ |
– |
– |
– |
+ |
– |
– |
|
Cumulospora sp. |
– |
– |
– |
– |
– |
– |
+ |
– |
– |
– |
– |
|
Cyphellophora sp. |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
+ |
|
Hormographiella sp. |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
+ |
|
*Monodictys pelagica (Johnson) E. B. G. Jones |
– |
– |
– |
+ |
– |
– |
– |
– |
– |
– |
– |
|
Stemphylium tomatonis E.G. Simmons, 2001 |
– |
– |
– |
– |
– |
– |
– |
– |
+ |
+ |
– |
|
Stachybotrys chartarum (Ehrenb.) S. Hughes, 1958 |
+ |
– |
– |
– |
– |
– |
– |
– |
+ |
+ |
– |
|
Teleomorph stages |
|||||||||||
|
Chaetomium globosum Kze, 1817 |
+ |
+ |
|
|
|
|
|
|
|
|
|
|
*Corollospora trifurcata (Höhnk) Kohlm,1962 |
– |
– |
+ |
– |
– |
– |
– |
– |
– |
– |
– |
|
*Corollospora maritima Werdermann, 1922 |
+ |
– |
+ |
– |
– |
– |
+ |
+ |
– |
– |
– |
|
*Corollospora sp. |
|
– |
– |
– |
– |
– |
+ |
– |
– |
– |
– |
|
*Haligena elaterophora Kohlm, 1961 |
– |
|
– |
+ |
– |
– |
– |
– |
– |
– |
– |
|
*Halosphaeriopsis mediosetigera (Cribb & J.W. Cribb) T.W. Johnson, 1958 |
– |
– |
+ |
– |
– |
– |
– |
– |
– |
– |
– |
|
*Remispora maritima Linder, 1944 |
– |
– |
– |
– |
– |
+ |
– |
– |
– |
– |
– |
|
*Torpedospora radiata Meyers, 1957 |
– |
– |
– |
– |
– |
+ |
– |
– |
– |
– |
– |
Remark: SG – mycocomplex on the valves of the Black sea scallop Flexopecten ponticus Bucquoy Dautzenberg & Dollfus 1889; K – mycocomplex on crab; ChDA – mycocomplex on Chapek – Dox agar medium; * – obligate marine species of micromycete.
.jpg)
Before an experiment on availability of fungi culture luminescence was kept under temperature of 18 ± 2ºC, adapting for 2 h to the experiment conditions. In the studies of cultural liquid sterile mixture of the brine (16 S‰) and fresh water in correlation 1 : 1 was a control; dish with clean medium was a control for fungi grown on ChDA medium. The studies were conducted at the day time under complete darkness. Biophysical parameters of luminescence in micromycetes and their complexes were registered at the laboratory complex IMBR RAS “Svet” [11]. Device complex included high voltage power pack (HV-22), luminescope, consisting of an acceptor of light emission (FEU – 71) and dark camera for an object, as well as registration device – digital interface. Into dark camera of the luminescope they put specially made cuvette of 50 cm3 volume for mechanical and chemical stimulation of the bioluminescent organisms. The cuvette is made of transparent organic glass, into which they placed control organisms and organisms under experiment. They used mechanical and chemical stimulations of mycelium or cultural liquid for studying the main luminescence characteristics: energy and duration of their signals. Mechanical stimulation was conducted by quick putting in marine water into cuvette with cultures. As a chemical reagent fresh water and ethyl alcohol in 0.96% concentration, administrated with syringe into cuvette were approbated [17].
III. Results and discussion
On the ChDA medium cultures of the facultative marine micromycetes anamorph stages with 4–6 weeks age have been received. Mycelium ability to emit light was revealed in species A. fumigatus, Acremonium sp. and in complex of A. alternata, Cyphellophora sp., Hormographiella sp. (Fig. 3). In this group of cultures only A. fumigatus species appeared to be active under effect of all types of stimulation, and characteristics of luminescence energy (E) were maximum (Table 2).
.jpg)
Table 2. Mycocomplex composition, or fungus species (Е – light-emission energy, 108 quantum·cm-2; Т – light-emission duration, s)
|
Mycocomplex composition, or fungus species |
Mechanical stimulation (the marine water) |
Chemical stimulation |
||||
|
Fresh water |
Ethyl alcohol |
|||||
|
Е |
Т |
Е |
Т |
Е |
Т |
|
|
Corollospora maritima, Corollospora sp., Cumulospora sp. |
1.77 ± 0.08 |
4.49 ± 0.21 |
3.44 ± 0.17 |
4.57 ± 0.22 |
0.48 ± 0.02 |
4.39 ± 0.21 |
|
Alternaria alternata, Cyphellophora sp., Hormographiella sp. |
1.14 ± 0.05 |
4.47± 0.22 |
2.24 ± 0.11 |
4.94 ± 0.24 |
– |
– |
|
Acremonium sp. |
1.12 ± 0.05 |
4.46 ± 0.22 |
3.35 ± 0.11 |
4.53 ± 0.22 |
– |
– |
|
Aspergillus fumigatus |
1.87 ± 0.08 |
4.59 ± 0.21 |
3.24 ± 0.11 |
4.96 ± 0.24 |
1.04 ± 0.05 |
4.38 ± 0.21 |
The cultures of Acremonium sp. and mycocomplex (A. alternata, Cyphellospora sp. and Hormographiella sp.) did not emit light under stimulation by alcohol. Fungi complex had less expressed light emission if compared with A. fumigatus and Acremonium sp. In Penicillium sp. and P. commune luminescent ability has not been revealed under such means of stimulation.
The second series of experiments was conducted with the cultural liquids of mycocomplexes consisting of anamorph and teleomorph stages of the obligate and facultative marine fungi species, isolated on the scallop valves, crab carapax and wood fragments. Liquid age was of 7 – 33 months. Positive result has been received only for the mycocomplex of C. maritima, Corollospora sp., Cumulospora sp., isolated on the wood fragments. The given complex reacted positively to all stimulation types. For all cultures able to emit light common peculiarity has been revealed: maximum average magnitudes of luminescence energy under chemical stimulation by the fresh water were more than under mechanical one 1.7 – 2.9 times. Duration of luminescence in all cultures under study remained to be relatively constant not depending on stimulation type, making averagely 4.35 – 4.96 s.
Comparison of typical luminescent signals of the mycocomplex C. maritima, Corollospora sp., Cumulospora sp. cultural liquid under mechanical stimulation and chemical way of the fungi luminescent system irritation by the fresh water has shown that their luminescence differed (Fig. 4). For example luminescent signal caused by marine water represents itself as a flash of average amplitude, which first decreases and then grows, reaching its amplitude maximum with 3.44 + 0.17·108 quantum·cm-2. Under mycocomplex chemical stimulation they observe one – two weak signals of small amplitude with sharp front of growing and with the same decrement of descending.
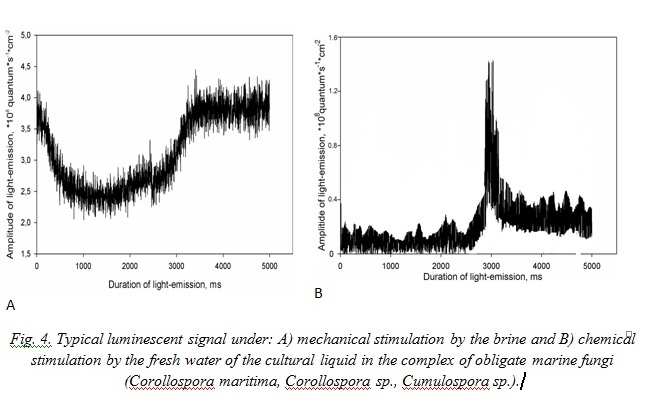
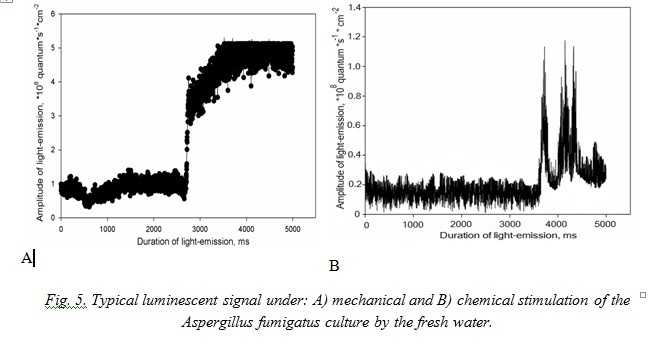
Typical signals of A. fumigatus under effect of the marine water represent non-considerable in amplitude flashes with sharp peak of growth with maximum at the third second after stimulation. Effect of the fresh water for mycomycetes is analogous to this in the mycocomplex C. maritima (Fig. 5).
According to the character of luminescence in the mycomycetes under study we can determine that culture signals with both types of stimulation are more durable than those we registered in the other bioluminescent species. Really, while majority of the known bioluminescent organisms (marine algae, coelenterates, ctenophores) emit light as impulses, fungi luminescence due to its character resembles bacteria luminescence [2, 13, 20].
Lighting mycocomplexes by the ultraviolet light on the ChDA medium, hydrobionts and wood fragments have shown that “old cultures” (5–6 weeks on ChDA, 7–33 months on another substrates) were fluorescent; on ChDA anamorph stages dominated in the species composition, marine species at the teleomorph stage dominated on hydrobionts and wood. It is also possible that like the land wood luminescent fungi from the Basidiomycota section, marine mycomycetes get ability to emit light after mycelium stops to grow, which takes place after starvation of substrate they grow on [1]. The ChDA medium dries and becomes poor soon that is why ascomata of fungi have no time for formation. Obviously in the cultural liquid without micromycetes there takes place decomposition of the substances, which provide an effect of luminescence that is why light emission has been revealed only in one variant of experiment. We suppose that character of the micromycetes luminescent response is determined by their trophic specialization, growth rate, different relation to the temperature and another factor. All this will be a subject of the further investigations.
1. Bioluminescent analysis of the molecular processes in cells and their physical-chemical models; Creation on their basis of a new generation of bioluminescent sensors for biology and medicine. Report about scientific-research work in the frame of the federal target program “Scientific and scientific - pedagogical cadres of the innovational Russia” for 2009 - 2013 (intermediate stage № 2). The title of the stage: “The studies of molecular - cell mechanism of the bioluminescent fungi”. Head of SRW, academician, doc. of med. sci., prof. I.I. Gitelzon. Krasnoyarsk, 2010, 87 pp.
2. I.I. Gitelzon, E.K. Rodicheva, S.E. Medvedeva, G.A. Primakova, S.I. Bartsev, G.A. Kratasyuk et al., Luminescent bacteria. Novosibirsk: Nauka, 1984, 386 pp.
3. S.H.D. Haddock, M.A. Moline, and J.F. Case, “Bioluminescence in the Sea,” Annu. Rev. Marine. Sci., vol. 2, pp. 443-493, 2010.
4. G.S. De Hoog, J. Guarro, J. Gene, and M.J. Figueras, Atlas of clinical fungi, 2rd ed., Centraalbureau voor Schimmelcultures, 2000, 1126 pp.
5. K.D. Hyde and S.B. Pointing, “Marine mycology”, A Practical Approach, Hong Kong: Fungal Diversity Press, 2000, 370 pp.
6. A.E. Ivanova and O.E. Marfenina, “Life - ability of mycelium fragments and vegetation of spores of some species of the microscopic fungi in different ecological conditions,” Modern problems of mycology, algology and phytopathology, pp. 216-217, 1998.
7. D. Khundzhua, E. Fedoseeva, S. Patsaeva, V. Terekhova, and V. Yuzhakov, “Spectroscopy of chromophoric organic substances released by soil microscopic fungi into aqueous medium,” Czech Republic, pp. 56-49, June 2011 [Digests 5th EARSeL Workshop on Remote Sensing of the Coastal Zone Prague, Czech Republic, p. 301, 2011].
8. D. Lapota, “Bioluminescence - Recent advances in oceanic measurements and laboratory applications,” in Tech Janeza Trdine, vol. 9, 2012, 190 pp.
9. Luminesent analysis. Eds. M.A. Konstantinova-Shlezinger, Moscow: The State editorial of physical-mathematical literature, 1961, 400 pp.
10. O.E. Marfenina, “Opportunistic fungi in the anthropogenically disturbed ecosystems,” Modern problems of mycology, algology and phytopathology, pp. 249-250, 1998.
11. O. Mashukova and Yu. Tokarev, “Variability of the bioluminescence characteristics of the Black Sea ctenophores-aliens in connection with different conditions of nutrition,” Open journal: Adv. Biosci. Biotechnol. (ABB), vol. 4, № 11, pp. 968-973, 2013. . http://www.scirp.org/journal/abb/
12. Methods of experimental mycology. Manual. Kiev: Nauk. Dumka, 1982, 552 pp.
13. J. Poupin, An.-Sop. Cussatlegras, and P. Geistdoerfer, “Plancton Marin Bioluminescent. Inventaire documenté des espèces et bilan des formes les plus communes de la mer d’Iroise,” Rapport scientifique du Loen, Laboratoire d’Océanographie de l’École Navale, LOEN, 1999, 64 pp.
14. K.L. Rypien, J.P. Andras, and C.D. Harvell, “Gliobally panmictic population structure in the opportunistic fungal pathogen Aspergillus sydowii”, Molecular Ecology, vol. 17, № 18, pp. 4068-4078, 2008.
15. O. Shimomura, “Bioluminescence: Chemical principles and methods”, World Scientific, 2006, 470 pp.
16. H. Souheil, A. Vey, P. Thuet, and J.P. Trilles, “Pathogenic and toxic effects of Fusarium oxysporum (Schlecht.) on survival and osmoregulatory capacity of Penaeus japonicus (Bate)”, Aquaculture, vol. 178, № 3-4, pp. 209-224, 1999.
17. Yu.N. Tokarev, “The bases of the hydrobionts biophysical ecology”, Sevastopol: ECOSI-Hydrofizika, 2006, 342 pp.
18. E.S. Trepova and A.G. Goryaeva, “Bioluminescent method for determination of polymer materials infection. Complex checking of the book-storages”, Methodical manual, Composed by T.D. Velikova, Eds. E.G. Vershinina, Sankt-Peterburg, pp. 147-153, 2013.
19. E.S. Trepova and T.D. Velikova, “Express-method for determination of the documents degree of being invaded. Complex checking of the book-storages”, Methodic manual, Composed by T.D. Velikova, Eds. E.G. Vershinina, Sankt-Petersburg, pp. 142-146, 2013.
20. Y.D. Tsviatkova, D.I. Voropaeva, and O.V. Povorova, “Cultivation and study of morphological and biochemical features of the luminescent bacterias”, International Research Journal, Biological Scienses, vol. 5-1 (12), pp. 54-56, 2013.
21. Wenli Chen, Da Xing, Weigui Chen, and Yingliang Li, “View Author Affiliations Changes of delayed luminescence spectra in rice of different polluted degree by Aspergillus flavus”, Chinese Optics Letters, vol. 3, Issue S1, pp. S188-S190, 2005.
22. Luminescent analysis of the food products http://biobloc.ru/lyuminescentnyy_analiz__pischevyh_p
23. Optical methods of researches. Electronic document. http://veterinary.academic.ru/3473







