BISAC NAT010000 Ecology
BISAC NAT045050 Ecosystems & Habitats / Coastal Regions & Shorelines
BISAC NAT025000 Ecosystems & Habitats / Oceans & Seas
BISAC NAT045030 Ecosystems & Habitats / Polar Regions
BISAC SCI081000 Earth Sciences / Hydrology
BISAC SCI092000 Global Warming & Climate Change
BISAC SCI020000 Life Sciences / Ecology
BISAC SCI039000 Life Sciences / Marine Biology
BISAC SOC053000 Regional Studies
BISAC TEC060000 Marine & Naval
A 20% increase of the carbon dioxide concentration in the atmosphere during the last century and a dramatic increase in nutrient load to marine systems due to human activity have resulted in pronounced carbon cycle transformation in coastal areas. Acidification and carbon dioxide increasing in the water column and appearance of oxygen minimum zones are reported for the worldwide coast. This makes ecological assessment of aquatic systems, including key cycles of elements, an important social and scientific task. In this study, we present information on the inorganic part of the carbon cycle and its transformation in the Sevastopol Bay (the Black Sea). This semi-enclosed coastal area has been under heavy anthropogenic pressure over the last century. Municipal and industrial sewage discharge, maritime activities, including excavation of bottom sediments, provide additional sources of nutrients and organic carbon. We present data on dynamics of the inorganic part of the carbon cycle from 1998 – 2015. Values of pH and total alkalinity were obtained analytically, whereas CO2, HCO3-, CO32- concentrations and pCO2 values were calculated. Dissolved inorganic carbon (DIC) and its partitioning into CO2, HCO3-, CO32- demonstrate the state of the carbon cycle and its evolution. Our observations reveal up to 2% increase of DIC from 1998 – 2015, but the value of pCO2 has increased by up to 20% in line with declining pH (acidification). Seasonal variations are far more pronounced and reveal extremes for areas of oxygen minimum zones. This results in negative consequences for the ecosystem, but these consequences for the Sevastopol Bay’s ecosystem remain reversible and the carbonate system can be restored to its natural state.
carbonate system transformation, pCO2, Sevastopol Bay
- INTRODUCTION
Coastal areas, being the major link between terrestrial and marine ecosystems, are under keen interest of scientists. Coastal ecosystems reveal results of natural and anthropogenic activities, and they expose fast deviations from their nature state. This is the reason of investigations of coastal systems and their stability under climate variations and anthropogenic pressure.
Long-term observations show reversible changes in marine ecosystems even under significant anthropogenic pressures. This resistance is a result of the system’s buffer capacity, allowing restoring the original quality of the ecosystem, supporting its stability and recovering. One of the most important marine buffer systems is the carbonate system, which is the key part of the biogeochemical carbon cycle. The carbonate system mainly determines pH consistency [1]. Any deviations in pH, e.g. acidification, leading to negative consequences of appearance of reduced heavy metals and other toxic species in the water column, the dissolution of coccolithophores and mollusk carbonate shells, coral reefs and carbonate saturated sediments [2].
The carbonate system, including carbonate- and bicarbonate-ions, dissolved carbon dioxide, carbonic acid and hydrogen ions, is highly sensitive to any changes in biogeochemical processes and physics occurring in coastal marine ecosystems [3]. Changes in temperature, pressure and salinity influence inorganic carbon partitioning. Organic carbon mineralization as well as its production result in changes in the total inorganic carbon concentration in the water column [1].
The global temperature has been increasing and carbon dioxide concentration in the atmosphere has increased more than 20% during the last century [4]. Euthrophication, caused by dramatic human activity induced increase in the nutrient and organic carbon input to marine systems, have also resulted in pronounced transformation of the carbon cycle in coastal areas [5]. Acidification, increasing carbon dioxide concentration in the water column and appearance of oxygen minimum zones have been reported for the worldwide coast. This makes ecological assessment of aquatic systems, including key cycles of elements, an important social and scientific task.
In this study, we present information on the inorganic part of the carbon cycle and its transformation in the Sevastopol Bay (Fig. 1). This is semi-enclosed estuarine type bay with entering river Chernaya. The bay has been under heavy anthropogenic pressure over the last century. Municipal and industrial sewage waters, maritime activities, including excavation of bottom sediments, provide additional sources of nutrients and organic carbon. Artificial sea-malls at the bay enter restrict water exchange with the open sea and support accumulation of organic carbon, leading to negative consequences for the bay’s ecosystem [6].
This work is addressed to dynamics of the inorganic part of the carbon cycle from 1998 – 2015.
- MATERIALS AND METHODS
Samples were collected from the surface and bottom layers of the Sevastopol Bay (Fig. 1) with Niskin bottles during 1998 – 2015.
.jpg)
Fig. 1. Sampling locations in the Bay of Sevastopol
The dissolved oxygen concentration, salinity, pH and alkalinity values were obtained analytically [7]. The total alkalinity was determined by direct titration by hydrochloric acid with a potentiometric end-point detection, pH was determined with a potentiometric pH-meter with NBS buffer solutions [1]. The standard deviation of measurement of 10 parallel samples did not exceed 0.02 pH units. Whereas CO2, HCO3-, CO32- concentrations and pCO2 values were calculated as suggested in [7]. The dissociation constants of carbonic acid, recommended by the Department of Marine Sciences of UNESCO, were used [7]. Boron was assumed a conservative part of salinity; therefore, the boron content was calculated from the salinity [4]. The effect of dissociation of water, phosphoric acid, sulfuric acid, hydrofluoric acid and other acids were not taken into account.
Data from the 1st station (Fig. 1) were not taken into account because it represented fresh waters of river Chernaya, rather than the considered marine waters of the bay.
- RESULTS AND DISCUSSION
The dissolved part of the carbonate system is represented by the system of reversible processes:
CO2 (atm) ↔ CO2 (water) ↔ CO2 + H2O↔ HCO3- + H + ↔ CO3 2- + 2H +
The total amount of dissolved CO2, HCO3- and CO32- is called dissolved inorganic carbon (DIC):
DIC = [CO2] + [HCO3-] + [CO32-] 1
DIC can serve as an indicator of changes in the carbon cycle [8].
Due to DIC is the sum of inorganic forms of carbon, its changes are governed by processes of photosynthesis (2) and CO2 exchange in the surface waters and organic carbon oxidation in the bottom waters (3).
6СО2 + 6Н2О → 6H+ + 6HCO3 - → С6Н12О6 + 6О2 2
С6Н12О6 + 6О2 ↔ 6СО2 + 6Н2О ↔ 6H+ + 6HCO3 - . 3
Photosynthesis results in consumption of inorganic carbon and production of organic carbon. This decreases DIC and increases pH (due to pH = –
In the Sevastopol bay’s waters, the content of DIC in the bottom layer has always been insignificantly higher as compared to the surface layer (Fig. 2), due to organic carbon oxidation. Our previous studies show that the content of organic carbon in sediments of the Sevastopol bay exceeds 4% [8] due to high anthropogenic pressure and accumulation of organic carbon in the bottom sediments. In spite of higher DIC concentrations in bottom waters, its changes from 1998 to 2015 do not exceed 1%, whereas this increase is about 2% in the surface layer (Fig. 2). This suggests that the flux of organic carbon to the bottom waters exceeds the oxidation potential and it increases the content of organic carbon in sediments.
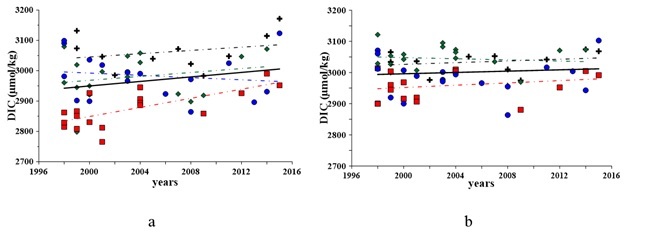
Fig. 2. Variations in the DIC in 1998 – 2015 in the surface (a) and bottom (b) layers of water: black cross – Winter, green rhomb – Spring, red square – Summer, blue circle – Fall
Seasonal DIC changes are more pronounced (Fig. 3). In cold seasons DIC concentrations expose maximum values, and winter DIC inventory in the surface layer is larger than in the bottom layer. This fact evidences good vertical ventilation of the entire water column and increasing CO2 solubility in cool waters. In warmer seasons, the DIC content in the bottom waters was larger than in the surface layer.
In spring, one can expect minimum DIC inventory in the surface layer due to active spring bloom of phytoplankton. Still, our observations reveal opposite results (Fig. 3). This might be due to seasonal flood of river Chernaya, supplying rich with organic matter, carbon dioxide and suspended matter waters.
A linear decrease of the DIC content occurs in the surface layer from winter to summer (Fig. 3). This fact can result from intensive photosynthesis, decreasing CO2 solubility, and strong vertical stratification, preserving carbon dioxide in bottom waters.
In fall, the DIC content raises. In the surface layer, its concentration equals spring’s values. This increase of DIC is a result of good vertical ventilation supporting contribution from the bottom layer and equal DIC concentrations in the surface and bottom layer (Fig. 3).
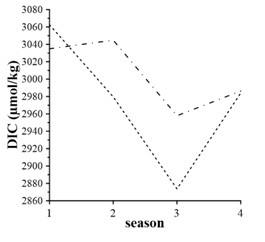
Fig. 3. Seasonal DIC variability averaged for 1998 – 2015: dash line – surface layer, dash dot line – bottom layer; numeric 1 – Winter, 2 – Spring, 3 – Summer, 4 – Fall
Seasonal variations of the DIC content in the bottom waters are also distinct, but they differ from those for the surface waters (Fig. 3). The maximum of DIC is in spring, but its value is insignificantly larger than in winter. In summer, strong seasonal stratification restricts oxygen supply to bottom waters. As the result, the oxygen flux is not enough for oxidation all of organic carbon (3), and this is the reason of organic carbon accumulation in bottom sediments. The DIC content increases in fall (Fig.3), and equals values in the surface layer, due to water mixing. Increased values of the DIC content in fall is likely due to accumulation of products of organic carbon oxidation.
Despite large seasonal variability, the overall average annual content of DIC in the water column for 1998 – 2015 does not reveal significant changes and/or persistent trends, which is the evidence of the bay’s carbonate system to recover.
The main contribution to DIC brings bicarbonate-ion (90%) and it has the same dynamics. In 1998 – 2015, an increase in its concentration has been observed. For the bottom layer, the bicarbonate-ion’s concentration has increased by about 1%. The content of carbon dioxide has decreased by 2% and the carbonate-ion concentration has risen by up to 2%. These changes are statistically significant and confirm our assumption of increasing contribution of organic carbon to the system and its increasing preservation in the bottom sediments.
For the surface waters, the revealed increase in the content of bicarbonate-ion is about 4%. The carbon dioxide concentration has increased in the surface layer waters by up to 20% and the concentration of carbonate-ion has decreased by 17%. The traced increase in the carbon dioxide concentration and nutrient supply to the Sevastopol bay’s support eutrophication and intensive production of organic carbon (2). We do not trace increasing DIC and HCO3-concentrations in the bottom waters, so we suppose that organic carbon is accumulated in sediments. This conclusion agrees with our previous investigations of bottom sediments of the bay [6, 8], revealing seasonal oxygen deficiency, reduced sulfur forms appearance and reduced conditions in the bottom waters and in the upper layer of the sediments.
An increase in the bicarbonate-ion concentration is accompanied by increase in the hydrogen ion concentration, which is revealed by descending values of pH (acidification). For 2009 – 2015, the average values of pH has lowered by 1% in the surface layer, and by 0.1% – in the bottom waters.
The traced decrease of pH in the bottom waters evidences a growth of organic carbon in the Sevastopol bay. As a result, CO2 is released to the water column and carbonates are gradually dissolved. The bottom waters, enriched in carbon dioxide, get mixed with the surface waters, providing additional CO2 and supporting primary production. As the content of CO2 in the bottom waters increases, the partial pressure rises too (4):
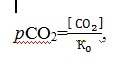 4
4
where [CO2] – the equilibrium concentration of carbon dioxide, µmol/l;
K0 – Henry constant for carbon dioxide, µmol/(l∙atm).
Photosynthesis (2) results in carbon dioxide uptake, DIC and pCO2 decrease and pH increase, which is accompanied by a shift in the carbonate system reducing the HCO3- concentration. Nutrients facilitate primary production processes in the surface waters, thus pH is expected to increase too. However, a decrease of pH, increase of the DIC and pCO2 content is found out in the surface layer (Fig. 2, 4). This may indicate that both photosynthesis and organic matter mineralization are the main biological and chemical processes, governing the carbonate system in the Sevastopol bay.
The carbon dioxide partial pressure in the bottom waters was expectedly above its level in the surface waters (Fig. 4). It is due to contribution of organic matter oxidation, produced in the bay’s waters (2) and/or provided from anthropogenic sources.
An increase of the pCO2 content in the bay’s waters has been observed for 1998 – 2015. From 2009 to 2015, the rate of this increase is higher in the surface layer than in the bottom layer (Fig. 4). For the surface waters, the noticed increase is about 17% or 50 µmol in the absolute value from 1998 to 2015, whereas it is 2% or 8 µmol in the bottom waters (fig. 4).
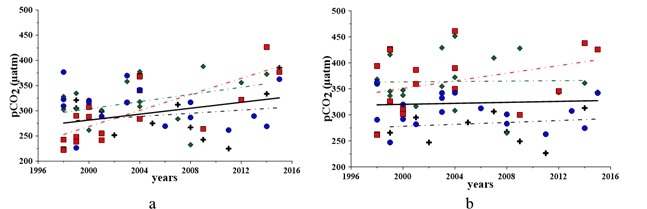
Fig. 4. pCO2 variations in the surface (a) and bottom (b) waters for 1998 – 2015: black cross – Winter, green rhomb – Spring, red square – Summer, blue circle – Fall
Seasonal variations of pCO2 (Fig. 4a) in the surface waters reveal a persistent upward trend in winter (by 9%), spring (by 19%) and especially in summer (up to 46%). This might indicate that the anthropogenic influence is stronger in summer. In spring and winter, intensive water dynamics and mixing balance the effects of eutrophication.
In the bottom layer (Fig. 4b), the maximum inter-annual uprising trend is traced for summer (by 17%). In winter and spring, this upward trend is about 1%. In fall, a 6% decrease of the pCO2 level has been observed.
Average seasonal variations in 1998 – 2015 (Fig. 5) demonstrate that surface pCO2 values are higher than the bottom values only in winter and they are minimum. It is due to good vertical mixing and slowdown of processes of organic carbon oxidation, in spite of increasing of gas solubility at low temperatures and absorption of CO2 from the atmosphere. In spring, the pCO2 level in the surface layer is smaller than in the bottom waters (Fig. 5). In the surface layer, minimum pCO2 values are due to spring phytoplankton bloom, which should lead to consumption of CO2 and decrease of pCO2, respectively. Maximum pCO2 values in the bottom waters are due to active organic carbon oxidation in sediments.
In summer, the level of pCO2 in the surface layer drops (Fig. 5), supporting the maximum difference between values in surface and bottom layers. The summer value of pCO2 in the bottom waters is comparable to those in spring (Fig. 5). This is due to organic carbon oxidation (3) and dissolution of carbonate-ion due to acidification.
In fall, pCO2 increases in the surface waters and equals values for the bottom waters (Fig. 5). This is due to increasing solubility of CO2 in cooling waters and spring-to-fall accumulation of products of organic matter oxidation. Carbon dioxide and nutrients, produced after organic matter oxidation, accumulate in the bottom waters and serve as an additional source of CO2 and nutrients for the surface layer.
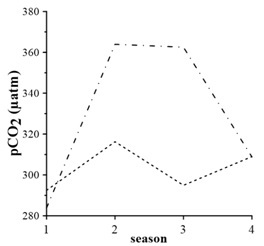
Fig. 5. pCO2 seasonal changes averaged for 1998 – 2015: dash line – surface layer, dash dot line – bottom layer; numeric 1 – Winter, 2 – Spring, 3 – Summer, 4 – Fall
- CONCLUSIONS
In warm seasons, the main processes governing the carbonate system in the water column are respiration of organic matter in sediments and bottom waters and photosynthesis in the surface layer. The surface waters still absorb carbon dioxide from the atmosphere, but this ability has decreased by 20% from 2001 to 2015. In cold seasons, water dynamics and mixing can still balance the effect of eutrophication and promote system’s recovery.
The content of DIC and pCO2 for 1998 – 2015 has increased. DIC changes are below 1% and statistically insign
Any changes in surface levels of pCO2 affect carbon dioxide exchange between water and the atmosphere. Depending on the concentration of CO2 in the surface layer the water column may serve as a source or sink of carbon dioxide.
Partial pressure of atmospheric carbon dioxide is currently about 400 µatm [10]. Thus, from our obtained data, waters of the Sebastopol bay usually absorb carbon dioxide from the atmosphere (Fig. 4a). The exception was summer of 2014, when the surface pCO2 reached 426 µatm and supported a flux of CO2 to the atmosphere. However, it is typical for coastal waters in summer [11].
Quantifying gas exchange between water and the atmosphere [12] our results show that invasion (when the water column absorb carbon dioxide from the atmosphere) is typical, but this ability to consume CO2 from the atmosphere has decreased by 20% from 2001 to 2015.
ificant. However, a significant increase of pCO2 in the bottom and the surface layers (2 and 17% respectively) in line with decreasing pH (acidification) have been traced, indicating anthropogenic pressure. Seasonal oscillations are far more pronounced, as compared to inter-annual trends, and reveal extremes for appearance of oxygen minimum zones. This results in negative consequences for the ecosystem, but these consequences for the Sevastopol Bay’s ecosystem remain reversible, and the carbonate buffer system can be restored yet. The ability of the bay to consumed CO2 from the atmosphere and preserve organic carbon in sediments will expire within the next few several years and the ecosystem will experience irreversible catastrophic changes.
This work has been funded from the RFBR research project №16-35-60006 mol_a_dk "Long-term changes in the carbon cycle characteristics of the Sevastopol bay."
1. R.E. Zeebe, D. Wolf-Gladrow, CO2 in seawater: equilibrium, kinetics, isotopes, Elsevier Oceanography Series, 2001, P. 346.
2. M. Bollmann et al., World ocean review. Living with the oceans. vol. 2. Maribus: Hamburg. 2010, P. 252.
3. R.A. Feely, S.C. Doney, and S.R. Cooley “Ocean Acidification: present conditions & Future changes in a high-CO2 World,” Oceanography, vol. 22, No.4, pp. 36-47, 2009.
4. F.J. Millero, “The Marine Inorganic Carbon Cycle,” Chemical Reviews, vol. 107, pp. 308-341, February 2007.
5. T. Kawano, “Trends in Ocean Acidification Research,” Science and Technology Trends, Quarterly Review, vol. 36, pp. 68-78, 2010.
6. N.A. Orekhova, S.K. Konovalov, “Polarography of the bottom sediments in the Sevastopol Bay,” Physical Oceanography, vol. 19, No.2, pp. 111-123, March 2009.
7. “Thermodynamic of the carbon dioxide system in seawater,” in Unesco technical papers in marine science, No.51, Unesco, 1987. pp. 3-21.
8. O.G. Moiseenko, N.A. Orekhova, “Investigation of the mechanism of the long-term evolution of the carbon cycle in the ecosystem of the Sevastopol bay,” Physical Oceanography, vol. 21, No.2, pp. 142-152, July 2011.
9. A.A. Bezborodov, V.N. Eremeev. Physical and chemical aspects of interaction between ocean and atmosphere, Kiev: Naukova Dumka, 1984, P. 192.
10. E. Dlugokencky, P. Tans, NOAA/ESRL (www.esrl.noaa.gov/gmd/ccgg/trends/)
11. J.E. Bauer, W.-J. Cai, P. A. Raymond, T. S. Bianchi, C. S. Hopkinson, and P. A. G. Regnier, “The changing carbon cycle of the coastal ocean,” Nature, vol. 504, pp. 61-70, 2013.
12. Y.I. Lyahin, V.P. Alexandrov, N.I. Pal’shin, “The calculation of CO2 exchange balance between the ocean and the atmosphere for the Atlantic Indian and Pacific Oceans,” Research and development of the World Ocean, Leningrad: Leningrad Hydrometeorological Institute, vol. 65, pp. 48-60, 1978.







