BISAC NAT010000 Ecology
BISAC NAT045050 Ecosystems & Habitats / Coastal Regions & Shorelines
BISAC NAT025000 Ecosystems & Habitats / Oceans & Seas
BISAC NAT045030 Ecosystems & Habitats / Polar Regions
BISAC SCI081000 Earth Sciences / Hydrology
BISAC SCI092000 Global Warming & Climate Change
BISAC SCI020000 Life Sciences / Ecology
BISAC SCI039000 Life Sciences / Marine Biology
BISAC SOC053000 Regional Studies
BISAC TEC060000 Marine & Naval
Polychlorinated biphenyls (PCBs) can still be a problem for the aquatic environment. Fish species are a suitable indicator for the environmental pollution monitoring because they concentrate pollutants in their tissues directly from water. Concentrations of PCBs were measured in marine fish, collected from Bulgarian Black Sea coast in order to monitor the dynamics of these pollutants in 2007, 2010 and 2015. The fish species: goby (Neogobius melanostomus), sprat (Sprattus sprattus sulinus), horse mackerel (Trachurus Mediterraneus ponticus) and grey mullet (Mugil cephalus) were chosen because of their characteristic feeding behavior. The PCBs were determined by gas chromatography system with mass spectrometry detection. The Total PCBs ranged from 93.8 to 513.3 ng/g lipid weight (in grey mullet and goby, respectively). Levels of PCBs in goby and grey mullet decreased in 2010 and 2015. In order to assess the safety of fish as food were calculated TEQ. They are determined by the results of dioxin - like (dl) PCBs. TEQs were calculated from 0.01 to 0.04 pg TEQ/g ww and did not exceed the EC limit of 3 pg TEQ/g ww. The levels of PCBs in fish from Bulgarian Black Sea were comparable to those found in neighboring seas.
PCBs, fish, risk assessment, Black Sea, Bulgaria.
I. Introduction
Polychlorinated biphenyls (PCBs) were listed as persistent organic pollutants (POPs) in the Stockholm Convention [1]. These chemicals are lipophilic, persistent, and accumulate through the food chain. The marine environment is particularly vulnerable to POPs because it acts as the final sink and consequently contains the major portion of these compounds [2].
The European Union Water Framework Directive (WFD) lays down a strategy against pollution all European waters (rivers, lakes, ground and coastal waters) and the Environmental Quality Standards (EQS) for 33 priority substances and other 8 pollutants with the aim of achieving a good chemical status of surface water [3, 4]. The EQS is set for prey tissue (wet weight) and member states being able to choose “the most appropriate indicator from among fish, molluscs, crustaceans and other biota” [3]. Fish are used extensively for environmental monitoring, because they uptake contaminants directly from water and diet [5,6]. Generally, the ability of fish to metabolize organochlorines is moderate; therefore, contaminant loading in fish is well reflective of the state of pollution in surrounding environments [7]. Data on the presence and distribution of organochlorine contaminants in fish species are therefore important from ecological perspective [8].
The contamination of PCBs is a significant health problem because these compounds can cause several adverse effects to human health and wildlife survival [7,9]. For these reasons, most countries have restricted the use of PCBs since 1970s. PCBs are a group of chemicals primarily used in transformers, capacitors, paints and printing inks, and also in many other industrial applications.
The Black Sea is the world largest land-locked sea. The north-western area is subject to the discharge of large rivers (the Danube, Dnieper and Dniester). The Black Sea is bordered by Bulgaria and Romania to the west, Ukraine to the north, the Russian Federation and Georgia to the east and Turkey to the south [10]. In the past, the Black Sea has been threatened with problems from chlorinated compounds as well as other chemical pollutants [11]. PCBs were investigated in sediments and waters from north-western Black Sea coastal areas [10,12].
The purpose of this study was to determine the levels of polychlorinated byphenils in four fish species: goby, sprat, horse mackerel and grey mullet from the Bulgarian Black Sea coast and to monitor the presence and dynamic of these pollutants during the 2007 – 2015.
II. Materials and methods
Sampling and sample preparation
The fish species were selected according to their characteristic feeding behaviour and importance to human consumption in Bulgaria: goby (Neogobius melanostomus), sprat (Sprattus sprattus sulinus), horse mackerel (Trachurus Mediterraneus ponticus) and grey mullet (Mugil cephalus). Fish goby is non-migratory species and feed mainly with benthic organisms. Sprat is a small, pelagic marine fish - it feeds on planktonic crustaceans. Horse mackerel is pelagic, summer spawning fish with Mediterranean origin; it feeds with different small fish (anchovy, sprat), crustaceans and worms. Grey mullet is an omnivorous and detritivorous scavenger feeding mainly on organic matter as well as plankton, benthic organisms and algae.
Samples were collected from Black Sea coast of Bulgaria in 2007, 2010 and 2015. The samples were transferred immediately to the laboratory in foam boxes filled with ice and were stored in a freezer (-18oC) until analysis. The length and weight of each specimen were measured and rinsed with distilled water to remove sand and impurities. Biometric data for the various fish analyzed in this study is shown in Table 1.
Table 1. Biometric data of fish from Bulgarian Black Sea coast
|
Fish species |
n |
Lipids, % |
Length, cm |
Weight, g |
|
Goby (Neogobius melanostomus) |
12 |
2.6±0.3 |
16.0 ± 2.3 |
60.3 ± 17 |
|
Sprat (Sprattus sprattus) |
10 |
8.9±0.9 |
8.5 ± 1.1 |
4.4 ± 0.7 |
|
Horse mackerel (Trachurus mediterraneus ponticus) |
11 |
15.6±1.7 |
12.9 ± 2.0 |
21.2 ± 12 |
|
Grey mullet (Mugil cephalus) |
10 |
9.2±0.8 |
32.0 ±2.5 |
351 ±9.6 |
n – number of samples
Each sample was prepared from soft tissue of several individual fish of similar sizes. The number of fish collected in every sample varied between 30 and 40 for sprat and goby, between 20 and 25 for horse mackerel, and between 5 and 10 for grey mullet. The edible portion of the same species was homogenized using a blender; pools of about 300 g were made with fillets taken from several individual fish.
Chemical analysis
Samples were prepared according to a previously described method [13]. Twenty grams of homogenized fish tissue were mixed with 100 g of anhydrous sodium sulfate and was extracted with hexane / dichloromethane (3/1, v/v) in Soxhlet extractor for 16 h. Each sample was spiked with internal standards PCB 30 and PCB 204. The solvent was carefully evaporated and the lipid content was determined gravimetrically of an aliquot of the extract (1/5th). The extract was cleaned-up on a glass column (10x250 mm) packed with 2 g neutral silica, 4 g acid silica and 2 g neutral silica (Merck KGaA, Darmstadt, Germany). PCBs were eluted with 50 ml n-hexane (Sigma-Aldrich Chemie, Taufkirchen, Germany). The eluates were concentrated to near dryness and reconstituted in 0.5 ml in hexane. One micro liter of purified extract was injected into GC/MS.
Gas chromatographic analysis of PCBs were carried out by GC FOCUS (Thermo Electron Corporation, Austin, Texas, USA) using POLARIS Q Ion Trap mass spectrometer. Experimental MS parameters are the following: Ion source and Transfer line temperatures were 220oC and 250oC, respectively. The splitless Injector temperature was 250oC. The PCBs experimental temperature program was 90oC for 1 min, then programmed 30oC/min to 180oC, 2oC/min to 270oC, 30oC/min to 290oC with a final hold for 3.0 min. Splitless injections of 1 μl were performed using a TR-5MS capillary column (Bellefonte, PA, USA) with a length of 30 m, 0.25 mm ID and a film thickness of 0.25 μm. Helium was applied as carrier gas at a flow of 1 ml/min.
Pure reference standard solution PCB Mix 20 (Dr. Ehrenstorfer Laboratory, Augsburg, Germany) was used for instrument calibration, recovery determination and quantification of compounds. Measured compounds were PCB congeners: IUPAC № 28, 31, 52, 77, 101, 105, 118, 126, 128, 138, 153, 156, 169, 170, 180. The limits of quantification (LOQ) varied for individual PCBs from 0.2 to 0.5 ng/g lw. Each sample was analyzed three times and was taken an average of the results obtained.
Quality control
The quality control was performed by regular analyses of procedural blanks and certified reference material BB350 (PCBs in Fish oil) – Institute for Reference Materials and Measurements, European Commission. PCBs recovery from certified reference material varied in the range 85 -106% for individual congeners. Procedural blanks and a spiked sample with standards were analyzed between each 5 samples to monitor possible laboratory contamination. Blanks did not contain traces of contaminants.
III. Results and discussion
The six indicator PCBs (IUPAC No 28, 52, 101, 138, 153, 180) are recommended by the European Union for assessing the pollution by PCBs [14]. The existence of indicator PCBs is important for the prediction of degree of lipophilic contamination although their toxicity is less than that of dioxin-like PCBs. The mean concentrations of Indicator PCB congeners in investigated fish species collected from the Black Sea in 2015 are present in Table 2.
Table 2 Concentrations of Indicator PCBs (ng/g lipid weight, mean values and standard deviation) determined in fish collected from the Black Sea in 2015.
|
Species |
n |
PCB 28 |
PCB 52 |
PCB 101 |
PCB 138 |
PCB 153 |
PCB 180 |
|
goby |
12 |
42.4±12.6 |
13.1±3.1 |
14.6±4.4 |
46.8±13.5 |
61.9±19.2 |
32.0±10.1 |
|
sprat |
10 |
19.2±5.8 |
22.8±7.1 |
20.2±6.2 |
36.2±21.2 |
46.9±11.9 |
28.9±9.8 |
|
horse mackerel |
11 |
7.8±2.6 |
7.8±3.1 |
7.9±3.6 |
19.5±6.2 |
25.8±8.2 |
9.8±3.2 |
|
grey mullet |
10 |
28.0±6.9 |
6.5±2.3 |
<0.2 |
24.3±6.7 |
28.6±8.7 |
12.3±2.8 |
n - number of samples
The PCB patterns were always dominated by a large contribution from the hepta- and hexachlorinated PCBs 180, 153 and 138 in all investigated species (Table 2). The predominance of hexachlorinated PCBs in marine fish species, especially PCB 153 and PCB 138, has been reported by several authors for different coastal areas in Marmara Sea [15], in the Adriatic Sea [16,17] and in the Mediterranean [18].
The concentration levels of individual PCBs congeners in fish species sampled in 2007 and in 2010 were described in our previous study [13,19]. The data of our previous studies were summarized and the Sum of six I-PCBs congeners (mean values) was presented for fish species investigated in order to compare the results to the levels of PCBs in 2015. The concentration ranges of the annual means of Total PCBs measured in fish species in 2007, 2010 and 2015 are shown in Figure 1.
The differences in feeding preferences and lipid content of goby, sprat, horse mackerel and grey mullet justify the large range of observed PCBs levels. Actually, in marine organisms, habitat, physiologic factors, lipid content, geographical origin and feeding behaviour, are significant aspects that explain pollutants accumulations and pollutants elimination [20].
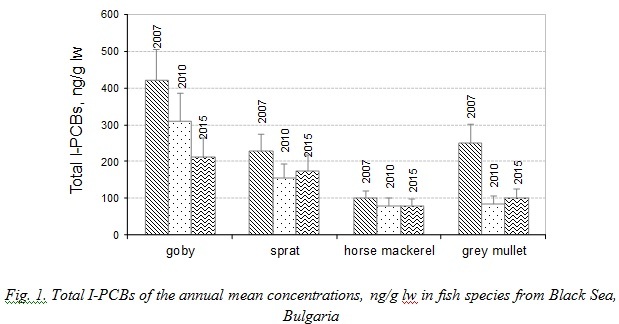
Total Indicator PCBs were found in all marine fish at concentrations ranging between 78.6 ng/g lipid weigh (lw) (horse mackerel 2015) and 420.5 ng/g lw (goby 2007). The statistical test on PCBs levels indicated that the differences among average annual concentrations were statistically significant (p<0.05) in goby and grey mullet. Levels of PCBs in goby and grey mullet decreased in 2010 and 2015. The experimental results showed no significant differences between mean annual sum of I-PCBs in sprat and horse mackerel sampled in 2007, 2010 and 2015.
The lowest PCBs concentrations were observed in horse mackerel. Due to its low trophic position horse mackerel possibly accumulates relatively small amounts of organochlorines. The statistical test indicated that the levels of PCBs found in goby were significantly higher than those detected in horse mackerel (p<0.05, t=0.001). Horse mackerel migrate from the Marmara Sea into the Black Sea in summer. This behavior makes interpreting contaminant levels in migratory fish difficult regarding different geographical environments. Consequently, these species could not be expected to offer a reliable picture of the level of pollution at the site where they are caught.
The toxic ‘‘dioxin-like’’ PCB congeners (IUPAC No 77, 105, 118, 156, 126, 169) showed the lowest levels, especially the non-ortho congeners (PCBs 77, 126, and 169) with concentrations below LOD for most of the samples. The Total dl-PCBs concentrations (mean values) measured in fish species in 2015 are shown in Figure 2.
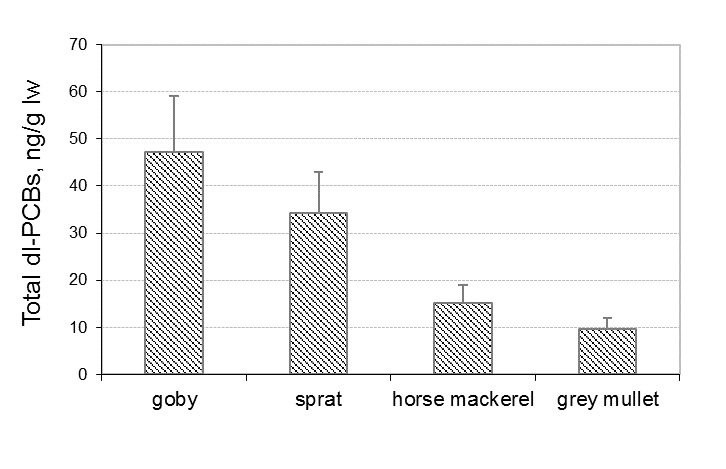
Fig. 2. Total dl-PCBs concentrations, ng/g lw in fish species from Black Sea, Bulgaria
As it is shown, the lowest Total dl-PCBs was found in grey mullet samples (mean 9.6 ng/g lw) and the highest results were observed in goby (mean 47.3 ng/g lw).
The concentration levels of dl-PCBs were determined in order to estimate the toxicity potential (TEQs) of PCB exposure. TEQ values were calculated by multiplying the individual congener levels measured in each sample with its toxic equivalency factors (TEF), established by the World Health Organization (WHO-TEF) [21]. In our study mean TEQs of the 6 “dioxin–like” congeners were calculated 0.03 pg TEQ/g wet weight for goby and 0.04, 0.02 and 0.01 pg TEQ/g ww for sprat, horse mackerel and grey mullet respectively. The comparison of our TEQ values in fish with those in the literature showed lower levels than the TEQs in sardine from Spanish Atlantic southwest coast (0.75 pg/g ww) [22] and lower than those in salmon from the Baltic Sea (12.6 pg/ g ww) [23]. The Total WHO-TEQ values measured in fish from southern Baltic Sea varied from 3.4 pg/g to 15.2 pg/g fresh weight [24].
The European Union has set a limit of 3.0 pg TEQ/g wet weight in muscle meat of fish for the sum of dioxin-like PCBs [14]. In our study TEQs of the six dl-PCBs for all investigated fish species did not exceed this limit.
Comparison with PCBs levels from other regions
The levels of PCBs detected in the fish species from the Bulgarian Black Sea were compared with those found in similar species from other marine ecosystems. The lowest level of the Total PCBs (like sum of 15 PCBs congeners) was found in horse mackerel 2015 (93.8 ng/g lw) and the highest level - in goby 2007 (513.3 ng/g lw). The experimental results indicate that PCB contamination of fish from the Bulgarian Black Sea coast is comparable to the results from the Marmara Sea, where average concentrations (sum of seven PCB congeners) were found in the range from 209.36 to 508.71 ng/g fat [15]. The levels of indicators PCBs found in present study are lower than the results of fish species from Gulf of Naples, the Mediterranean Sea (1005.3 - 17 259.0 ng/g lipid weight) reported by Reference [18]. Total PCB levels in goby from Belgian North Sea were found in the range 860 – 3200 ng/g lipid weight [25]. Our results were found lower than PCB concentrations in Atlantic mackerel (1096 ng/g lw) collected in the Central Adriatic Sea [17]. The low levels of PCBs observed in fish tissues correspond with the fact that no industrial production of PCBs in Bulgaria.
III. Conclusions
This study provides data on levels of PCBs contamination in marine fish species from the Bulgarian Black Sea coast. The mean levels of I-PCBs ranged between 85.6 ng/g lw (horse mackerel) and 313.6 ng/g lw (goby). Levels of PCBs in goby and grey mullet decreased in 2010 and 2015. Our results for sum of I-PCBs in all fish species did not exceed the maximum level of 75 ng/g ww. WHO-TEQs were found in the range from 0.01 pg TEQ/g ww to 0.04 pg TEQ/g ww (grey mullet and sprat, respectively) and did not exceed the limit of 3.0 pg WHO-TEQ/g ww for sum of dioxin-like PCBs. The contamination of the fish species investigated with PCBs appears to be relatively low compared to other European studies.
IV. Acknowledgment
This study was financed by EEA Grants and Ministry of Environment and Water of Bulgaria (Project D-33-49/2015).
1. UNEP, Stockholm Convention on Persistent Organic Pollutants (POPs). Available at: http://www.chem.unep.ch/sc/, 2001.
2. Tanabe, S., PCB problems in the future: Foresight from current knowledge. Environ Pollut 50, pp. 5-28, 1988.
3. European Commission, Directive 2008 /105/ EC of the European Parliament and of the Council of 16 December 2008 on Environmental Quality Standards in the Field of Water Policy, Off. J. Eur. Union, vol. L348, pp. 84, 2008.
4. L.I. Majoros, R. Lava, M. Ricci, B. Binici, F. Sandor, A. Held, H. Emons, Full method validation for the determination of hexachlorobenzene and hexachlorobutadiene in fish tissue by GC-ID MS. Talanta vol. 116, pp. 251-258, 2013
5. M.M. Storelli & G.O. Marcotrigiano, Occurrence and accumulation of organochlorine contaminants in swordfish from Mediterranean Sea: A case study. Chemosphere, vol. 62, pp. 375-380, 2006
6. R. Serrano, M. Barreda, M.A. Blanes, Investigating the presence of organochlorine pesticides and polychlorinated biphenyls in wild and farmed gilthead sea bream (Sparus aurata) from the Western Mediterranean Sea. Mar Pollut Bull, vol. 56, pp. 963-72, 2008.
7. A.T. Fisk, K.A. Hobson & R.J. Norstrom, Influence of chemical and biological factors on trophic transfer of persistent organic pollutants in the Northwater Polynya marine food web. Environ Sci and Technol, vol. 35, pp. 732-738, 2001.
8. K. Macgregor, I.W. Oliver, L. Harris, I.M. Ridgway, Persistent organic pollutants (PCB, DDT, HCH, HCB & BDE) in eels (Anguilla anguilla) in Scotland: current levels and temporal trends. Environ Pollut vol.158, pp. 2402 - 2411, 2010
9. J. Falandysz, B. Wyrzykowska, J. Warzocha, I. Barska, A. Garbacik-Wesolowska & P. Szefer, Organochlorine pesticides and PCBs in perch Perca fluviatilis from the Odra/Oder river estuary, Baltic Sea. Food Chem, vol. 87, pp. 17-23, 2004
10. G. Fillmann, J.W. Readman, I. Tolosa, J. Bartocci, J.-P. Villenueve, C. Cattini, L.D. Mee, Persistent organochlorine residues in sediments from the Black Sea, Mar Pollut Bull, vol. 44, 122-132, 2002.
11. G. Tuncer, T. Karakas, T.I. Balkas, C.F. Gokcay, S. Aygnn, C. Yurteri, G. Tuncel, Land-based sources of pollution along the black sea coast of Turkey: concentrations and annual loads to the Black Sea. Mar Pollut Bull, vol. 36, pp. 409-423, 1998.
12. C. Maldonado, J.M. Bayona, Organochlorine compounds in the north-western Black Sea water: distribution and water column process. Estuarine, Coastal and Shelf Sci, vol. 54, pp. 527-540, 2002
13. M. Stancheva, S. Georgieva, L. Makedonski, Organochlorine Pollutants in Fish from the Bulgarian Region of the Black Sea. Qual Assur and Safety of Foods and Crops, vol. 5(3), pp. 243 - 251, 2013
14. European Commission. Commission Regulation (EU) No 1259/2011 amending regulation (EC) no. 1881/2006 as regards maximum levels for dioxins, dioxin-like PCBs and non dioxin-like PCBs in foodstuffs. Off J. of the European Union, vol. L 320,pp. 18-23, 2011.
15. M. Coelhan, J. Stroheimer, & H. Barlas, Organochlorine levels in edible fish from the Marmara Sea, Turkey. Environ Intern, vol. 32, pp. 775-780, 2006.
16. S. Bayarri, L.T. Baldassarri, N. Iacovella, F. Ferrara, & A. di Domenico, PCDDs, PCFDs, PCBs and DDE in edible marine species from the Adriatic Sea. Chemosphere, vol. 43, pp. 601-610, 2001
17. M. Perugini, M. Lavaliere, A. Giammarino, P. Mazzone, V. Olivieri & M. Amorena, Levels of polychlorinated biphenyls and organochlorine pesticides in some edible marine organisms from the Central Adriatic Sea. Chemosphere, vol. 57, pp. 391-400, 2004.
18. B. Naso, D. Perrone, M.C. Ferrante, M. Bilancione, A. Lucidano, Persistent organic pollutants in edible marine species from the Gulf of Naples, southern Italy. Sci of The Tot Environ, vol. 343, pp. 83-95, 2005.
19. M. Stancheva, S. Georgieva, L. Makedonski, Polychlorinated biphenyls in fish from Black Sea, Bulgaria, Food Control, doihttps://doi.org/10.1016/j.foodcont.2016.05.012, in press
20. M. Van den Berg, L.S. Birnbaum, M. Denison, M. De Vito, W. Farland, M. Feeley, et al., The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci, vol. 93 (2), pp. 223-241, 2006
21. X. Bodiguel, V. Loizeau, A.-M. Le Guellec, F. Roupsard, X. Philippon, & C. Mellon-Duval, Influence of sex, maturity and reproduction on PCB and p,p′DDE concentrations and repartitions in the European hake (Merluccius merluccius, L.) from the Gulf of Lions (N.W. Mediterranean). Sci of The Tot Environ,vol. 408, pp. 304-311, 2009.
22. L.R. Bordajandi, I. Martin, E. Abad, J. Rivera, & M.J. Gonzalez, Organochlorine compounds (PCBs, PCDDs and PCDFs) in seafish and seafood from the Spanish Atlantic Southwest Coast. Chemosphere, vol. 64, pp.1450-1457, 2006.
23. P. Isosaari, A. Hallikainen, H. Kiviranta, P.J. Vuorinen, R. Parmanne, J. Koistinen, et al., Polychlorinated dibenzo-p-dioxins, dibenzofurans, biphenyls, naphthalenes and polybrominated diphenyl ethers in the edible fish caught from the Baltic Sea and lakes in Finland. Environ Pollut, vol. 141, pp. 213-225, 2006
24. J. Szlinder-Richert, I. Barska, Z. Usydus, W. Ruczyn´ska, & R. Grabic, Investigation of PCDD/Fs and dl-PCBs in fish from the southern Baltic Sea during the 2002-2006 period, Chemosphere, vol. 74, pp. 1509-1515, 2009
25. S. Voorspoels, A. Covaci, J. Maervoet, I. De Meester, & P. Schepens, Levels and profiles of PCBs and OCPs in marine benthic species from the Belgian North Sea and the Western Scheldt Estuary. Mar Pollut Bull, vol. 49, pp. 393-404, 2004







