from 26.12.2016 until now
from 24.12.2017 until now
BISAC NAT010000 Ecology
BISAC NAT045050 Ecosystems & Habitats / Coastal Regions & Shorelines
BISAC NAT025000 Ecosystems & Habitats / Oceans & Seas
BISAC NAT045030 Ecosystems & Habitats / Polar Regions
BISAC SCI081000 Earth Sciences / Hydrology
BISAC SCI092000 Global Warming & Climate Change
BISAC SCI020000 Life Sciences / Ecology
BISAC SCI039000 Life Sciences / Marine Biology
BISAC SOC053000 Regional Studies
BISAC TEC060000 Marine & Naval
Modern benthic foraminiferal assemblages in Alekseev Bay (Popov island), the Sea of Japan were studied during mariculture farming of Japanese scallop (Mizuhopecten yessoensis) and after its liquidation. Results of foraminiferal analysis pursued in period from 1985 to 2007 years show gradual changes in assemblage composition – increase of species richness from 86 to 107, number of agglutinated species from 2-3 up to 5-13 species and their distribution area under the mariculture. The most dominant families are Elphidiidae (Cribroelphidium frigidum, Protelphidium asterotuberculatum, Elphidium advenum depressulum), Discorbidae (Buccella frigida) .The most abundant agglutinated families are Trochamminidae i Ataxophragmiidae (Trochammina inflata, Eggerella advena).
mariculture, benthic foraminifera, assemblages, ecosystem recovery, the Sea of Japan.
I. Introduction
Humankind has been damaging the seas for decades by discharging pollutants into the water, destroying coastal ecosystems and overexploiting marine organisms. Aquafarming is known to change ecological condition of bays and coves, especially if it is done by bivalve outboard method. There are many research papers about changes in ecological structure of benthic communities in the conditions of mariculture in different marine ecosystems [1,2,3,4]. Suspended organic matter is reduced due to filtering processes by cultivated shellfish, thereby the concentration of fecal metabolites at the bottom is increased, which leads not only to its siltation, but also to the accumulation of organic matter in the soil [5]. This in turn leads to the bacterial growth, oxygen deficit, macrobenthos total abundance and number of species reduction, meiofauna population density increase [6]. Similar changes have occurred in the composition and distribution of benthic foraminifera in the Alekseev Bay studied under scallop farming over 10 years (1978-1988 years) and in the years after mariculture facilities removal [7,8,9]. Benthic foraminifera is a large group of protozoa with the body protected by shell. They are widely distributed in marine basins and found at all latitudes and depths of the oceans from the intertidal zone to the ultra depths. Among meiobenthic animals foraminifera may predominate both in number and biomass in many marine ecosystems, making it possible to work with relatively small samples to get statistically significant results [10]. Benthic foraminifera are a particularly useful group for environmental biomonitoring because they are highly sensitive to environmental perturbations [10, 11, 12].
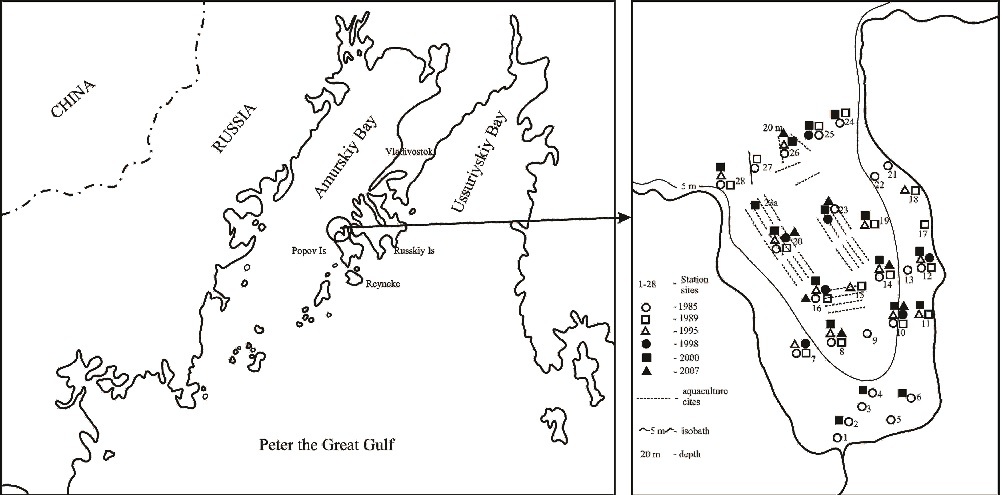
Fig. 1. Schematic map of the study area and the location of sampling stations collected in period of 1985-2007.
ii. Material and methods
Undisturbed sediment samples were collected at 28 stations in
Sediments were classified based on the dominant diameter of silt, sand, gravel, pebbles and biogenic particles present in the samples. Total content of particles different in size were determined using traditional nomenclature of particles [17]. The degree of bottom siltation was determined by calculating the average value of the fraction (<
iii. Results
This work is a result of long term research of benthic foraminifera composition and distribution in Alekseev bay (1978-1988), where for 10 years scallops were grown in cages, the young scallops were seeded over large areas, and in the last year mussels were also farmed in this area [4,7]. Creating populations of farmed aquatic organisms has led to ecosystem deterioration. It is known that the spatial and quantitative distribution of benthic foraminifera is closely related to the type of sediment, which is inhabited by animals [4, 20]. The ratio of bottom sediments siltation in the period used in mariculture Alekseev (1985) is 60%. After 12 years after the removal of the farm (2000), this figure fell to 39%. The granulometry results for sediments collected in 2007, also showed a decrease siltation of bottom sediments on the same stations for all years of study up to 34%. It also revealed a decrease in the Corg in sediments from 4-5% (1985) down to 0.9% (2000) and 1.2% (2007).
Significant changes occurred in foraminiferal assemblages in 12 years after the liquidation of the marine farm (2000). Species composition was changed: number of species increased, some species disappeared and many other species became widespread. There were 107 species belonging to 47 genera and 21 families. The most abundant families are the Elphidiidae, Discorbidae, Trochamminidae and Ataxophragmiidae. They were joined by representatives of other families - Buliminella elegantissima and Cribrononion incertus spread throughout the bay. The number of agglutinated species has risen up to 21. It should be noted that Rotaliammina ochracea, seen at certain stations before, has been found at almost all stations of the bay. Ammoscalaria fluvialis, Ammotium inflatum, Glomospira gordialis, Siphonaperta macbeathi, first appeared in the bay in 1998 also spread to several stations. The number of agglutinated forms at some stations increased to
After 19 years after the scallop mariculture liquidation in Alekseev bay (2007) 104 species belonging to 43 genera of 20 families were found. 10 species are the most abundant: Trochammina inflata, Eggerella advena, Quinqueloculina cf. quinquecarinata, Buccella frigida, Discorbis subaraucana, Cribroelphidium frigidum, Protelphidium asterotuberculatum, Elphidium advenum depressulum, Retroelphidium subgranulosum, Buliminella elegantissima (Fig. 2.). Secretion species dominanted. Agglutinated foraminifera ranged from 11 to 44%. The exception was the north-east part of the bay (station 23), where the greatest number of foraminifera (57%) with a sand wall were found at a depth of
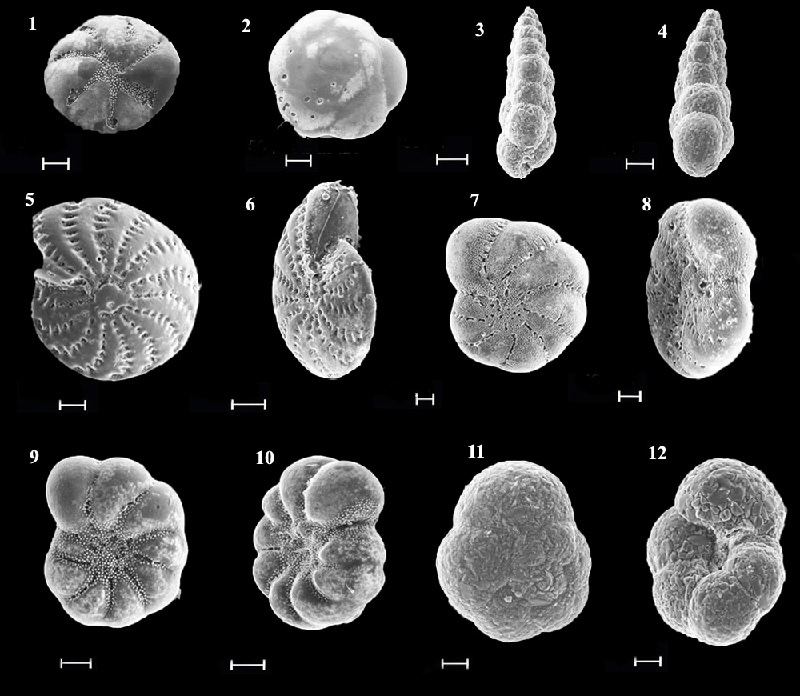
Fig.2. Electron images of representative foraminiferal species from Alekseev Bay: 1,2 – Buccella frigida; 3,4-Eggerella advena; 5,6-Elphidium advenum depressulum; 7,8 –Cribroelphidium frigidum; 9,10,- Protelphidium asterotuberculatum; 11,12 – Trochammina inflata,
scale bar 50 µm.
the highest (46%). P. asterotuberculatum was dominant, while E. advena was subdominant. The FD was similar on the other stations in the eastern and western parts of the bay (stations 8, 10, 16), and ranged from 288 to 304 thousand ind/m2. The highest number of agglutinating foraminifera in these areas was found at the station 10 (44%). T. inflata dominated, C. frigidum and E. advena were subdominant. C. frigidum prevailed at st. 8 and 16, P. asterotuberculatum, T. inflata and E. advena were the second most present. The percentage of living species ranged from 18 to 32%.
IV. Discussion
Results of the faunal analysis showed a gradual change in foraminiferal assemblages in the period from 1985 to 2007 as appeared in qualitative composition and distribution in the bottom sediments of the bay: increasing in species richness, structural reorganization of the assemblages, growing number of families and genera, agglutinated species and their distribution areas, increasing their numbers in the stations (from 2-3 to 5-10 species), which were previously located under the mariculture cages. Species richness also increased significantly at the stations as well where former mariculture cages were stationed. However FD decreased from year to year up until 2000, 12 years after the completion of marine farming, this figure increased to 600 thousand ind/m2, and S increased to
Table 1. Structural dynamics of foraminiferal assemblages in
|
Years |
1985 |
1989 |
1995 |
1998 |
2000 |
2007 |
|
Species richness (S) |
86 |
90 |
91 |
102 |
107 |
104 |
|
Number of families |
15 |
20 |
20 |
19 |
21 |
20 |
|
Number of genus |
35 |
42 |
41 |
41 |
47 |
43 |
|
Number of calcareous species |
73 |
76 |
77 |
87 |
86 |
84 |
|
Number of agglutinated species |
13 |
14 |
14 |
15 |
21 |
20 |
|
Average FD (thousand ind/m2) |
800 |
400 |
300 |
200 |
600 |
800 |
|
Average percent of living individuals |
11 |
26 |
30 |
36 |
32 |
28 |
The results of foraminiferal research allow us to recognize changes after 19 years after mariculture and shellfish cultivation in surveyed area levels of ecosystem. Granulometric structure of sediments gradually changed. The content of clay fractions in the soil at the same stations during 1985-2007 declined from an average of 64% (1985) to 39% (2000) and to 34% (2007). Apparently, this is one of the reasons that caused the structural reorganization in the foraminiferal assemblages. It showed in increasing species diversity, number of individual species, appearance of new species as well as a change of dominant and subdominant species of foraminifera. The area as well as FD of dominanated species of C. frigidum, B. frigida reduced after the mariculture farming. T. inflata, E. advena, P. asterotuberculatum become dominant and subdominant species at most stations in 2007, and prevailed only in a few stations in 1985 (Fig.3). There was a change in foraminiferal structure in
.jpg)
Fig.3. Percent ratio of the most representative species of Foraminifera in
Another reason for this change could be the disappearance of most groups of macrobenthos due to increased poaching in recent years. Visual observations by researchers from Marine Biology Institute and Pacific Oceanological Institute suggested that sea urchins, sea cucumbers, large clams, fish and even a few stars almost completely disappeared in
In coastal areas, which receive large amounts of organic matter, the number of common species can either decrease or increase, but species diversity is usually diminished. Other researchers also noted small number of species and low abundance of foraminifera directly in the areas of human impact. However, dramatic increase in species diversity was observed in few hundred meters of domestic waste [12]. Record values of FD of up to 6 million
1. Hargrave B.T., Duplisea D.E., Pfeiffer E., Wildish D. Seasonal changes in benthic fluxes of dissolved oxygen and ammonium associated with marine cultured Atlantic salmon. Mar. Ecol. Prog. Ser. V. 90. 1993, pp. 249-257.
2. Schafer C.T., Winters G.V., Scott D.B., Pocklington P., Cole F.E., Honig C. Survey of living foraminifera and polychaete populations at some Sanadian aquaculture sites: potential for impact mapping and monitoring. J. Foram. Res. V. 25, No. 3. 1995, pp. 236-259.
3. Scott D.B., Schafer C.T., Honig C., Younger D.C. Temporal variations of benthic foraminiferal assemblages under or near aquaculture operations - documentation of impact history. J. Foram. Res. V. 25, No 3. 1995, pp. 224-235.
4. Tarasova T.S. The effect of environment on the fauna of benthic foraminifers in near-shore ecosystems. V. 32, No. 2, 2006, pp. 85-94
5. Kucherjavenko A.V. Organic matter in shallow bays of Pos’et Bight. Vladivostok: TINRO-centr. 2002, pp. 86. [In Russian].
6. Van Colen, C., Meulepas, G., De Backer, A., van der Wal, D., Vincx, M., Degraer, S., Ysebaert,T.,. Diversity, trait displacements and shifts in assemblage structure of tidal flat deposit feeders along a gradient of hydrodynamic stress. Mar. Ecol. Prog. Ser. 406, 2010, pp. 79-89.
7. Tarasova T. S., Preobrazhenskaya T. V. Effect of the mariculture of the Japanese scallop on foraminifera assemblages in Alekseev Bight, Sea of Japan. Russian Journal of Marine biology. V. 26, № 3. 2000, pp. 166-174.
8. Tarasova T.S. Long-term variations in the composition and distribution of recent benthic Foraminifera in the northern part of Amursky Bay (Peter the Great Bay, Sea Japan). Ecological Studies and the State of the Ecosystem of Amursky Bay and the Estuarine Zone of the Razdolnaya River (Sea of Japan). Vladivostok: Dalnauka, V. 1, 2008, pp. 186 - 207.
9. Tarasova T.S., Zykova M.V., Pitruk D.L. Modern benthic foraminifera in the water area around the Peninsula Zhitkova (Amur Bay, Sea of Japan). Proceedings of the Russian-China bilateral symposium on marine ecosystem under the global change in Northwestern Pacific. Vladivostok, Russia, pp. 105-110. October 8-9. 2012
10. Gupta S.K. Modern Foraminifera. Kluwer Academic Publishers. 2002, pp 371.
11. Schafer C.T., Winters G.V., Scott D.B., Pocklington P., Cole F.E., Honig C. Survey of living foraminifera and polychaete populations at some Sanadian aquaculture sites: potential for impact mapping and monitoring. J. Foram. Res. V. 25, № 3. 1995, pp. 236-259.
12. Bresler V.M., Yanko-Hombach V.V. Chemical ecology of foraminifera. Parameters of health, environmental pathology, and assessment of environmental quality. Environmental micropaleontology. Ed. R. Martin. New York: Plenum. V. 15, 2000, pp. 217-247.
13. Saidova Kh.M., Major Distribution Patterns of Recent Benthic Foraminifera and Foraminifera Zones of the Pacific Ocean. Mode of Life and Distribution Patterns of Recent and Fossil Microfauna. Moscow: Nauka. 1975, pp. 62-70. [In Russian]
14. Loeblich A. R., Tappan H. N. Foraminiferal genera and their classification. Van Nostrand, Reinhold Co. New York. 1987, 1728 pp.
15. Shannon C.E., Weaver W. The mathematical theory of communication. Univ. Illinois Press. Urbana. 1964. 177 pp.
16. Gibson L.B. Some unifying characteristics of species diversity // Contrib. Cushman Found. Foram. Res. 1966. V. 17, No. 14. P. 117-124.
17. Parsons T.R., Takahashi M., Hargrave B.T. (1977) Biological Oceanographic Processes. Pergamon press, Oxford, 332pp.
18. Methods of chemical analysis in hydrological studies. Vladivostok: DVNC AN SSSR. 1979, pp. 51-55. [In Russian]
19. Pilotage northwestern Sea of Japan coast. GDNO USSR. 1984. pp. 122-123. [In Russian]
20. Debenay J.-P., Tsakiridis E., Soulard R., Grossel H. Factors determining the distribution of foraminiferal assemblages in Port Joinville Harbor (He d'Yeu, France): the influence of pollution // Mar. Micropaleontol. V. 43. 2001, pp. 75-118.
21. Sheremetevskij A.M. On the issue of macrobenthos compensation by meiobenthos on example of the White Sea mussel beds // Jekol. morja.. № 39. 1991, pp. 89-91. [In Russian].
22. Alve, E. Benthic foraminiferal responses to estuarine pollution: a review. Journal of Foraminiferal Research, v. 25, no. 3, 1995,pp. 190-203
23. Schafer C.T.; Collins E.S.; Smith J.N. Relationship of foraminifera and thecamoebian distributions to sediments contaminated by pulp mill effluent: Saguenay Fiord, Quebec, Canada. Mar. Micropal.V.17, 1991, pp. 255-283.
24. Martins V.A. et al. Response of benthic foraminifera to organic matter quantity and quality and bioavailable concentrations of metals in Aveiro Lagoon (Portugal). PLoS ONE. 2015; 10 (2): . doi:https://doi.org/10.1371/journal.pone.0118077 .
25. Angel, D. L., Verghese, S., Lee, J. J., Saleh, A. M., Zuber, D., Lindell, D., and Symons, A., , Impact of a net cage fish farm on the distribution of benthic foraminifera in the northern Gulf of Eilat (Aqaba, Red Sea). Journal of Foraminiferal Research, v. 30, 2000, pp. 54-65.







