from 26.12.2017 until now
from 26.12.2017 until now
from 26.12.2017 until now
from 26.12.2017 until now
BISAC NAT010000 Ecology
BISAC NAT045050 Ecosystems & Habitats / Coastal Regions & Shorelines
BISAC NAT025000 Ecosystems & Habitats / Oceans & Seas
BISAC NAT045030 Ecosystems & Habitats / Polar Regions
BISAC SCI081000 Earth Sciences / Hydrology
BISAC SCI092000 Global Warming & Climate Change
BISAC SCI020000 Life Sciences / Ecology
BISAC SCI039000 Life Sciences / Marine Biology
BISAC SOC053000 Regional Studies
BISAC TEC060000 Marine & Naval
There was suggested a method of obtaining a complex adsorbent with magnetic properties for the oil spill clean-up from the water surface by means of controlled magnetic field. As magnetic filler a finely-dispersed iron-ore concentrate in the form of magnetite, obtained by wet magnetic separation of crushed iron ore, was suggested. As an adsorbing component the disintegrating electric-furnace steelmaking slag, obtained by dry air-cooling method, was selected. The mass ratio of components slag:magnetite is 1(1,5÷2,0). For cleaning up emergency oil spills with the suggested magnetic adsorbent a facility, which is installed on a twin-hulled oil recovery vessel, was designed. The vessel contains a rectangular case between the vessel hulls with inlet and outlet for the treated water, the bottom of which is a permanently moving belt. Above the belt, at the end point of it there is an oil-gathering drum with magnetic system. The adsorbent is poured to oil-products layer from a hopper, provided with drum feeder. Due to the increased bulk weight the adsorbent sinks rapidly into the oil layer on the water surface. If the large non-floating flocculi are formed, they sink and sedimentate on the moving belt and are moved to the oil-gathering drum. The saturated adsorbent is removed from the drum surface with a scraper, connected with a gutter, with contains a rotating auger.
magnetic adsorbent, hydrophobization, magnetic field, oil products, structural changes, magnetite, steelmaking slag, oil recovery vessel.
I. Introduction
According to present-day ideas, oil and oil residues consist of low- and high-molecular hydrocarbonic and non-hydrocarbonic components [1]. By their colloid-chemical characteristics they are petrolic disperse systems of the complex internal structure, which can be altered under the influence of external factors [2].
The low-power technologies (acoustic, vibration, magnetic etc.), by means of which the structure of a material can be altered without significant external power consumption or by using its inner energy, are the most promising due to their effectiveness, economical efficiency and availability [3]. These methods are finding wider and wider application in the oil industry at the production, transportation and storage of high-viscosity and high pour point oils. Their usage allows achieving in a short period of time a significant level of destruction of petroleum associates, consisting of resins and asphaltine components and crystalline paraffin hydrocarbons, and sustaining this level within the time, necessary for mass-exchange processes [4],[5].
The application of sorption methods allows removing oil pollutions of a wide range of their nature to virtually any residual concentration independently of their chemical stability [6], [7]. And providing adsorbents with magnetic properties will make it possible to use physical methods of cleaning up oil spills by means of magnetic traps. So, designing an adsorbent with high sorption and magnetic properties which would allow its effective penetration into the oil products layer and rapid removal from the water surface with a controlled magnetic field is a relevant scientific and practical task.
II. Materials, conditions and methods of research
The authors have researched the sorption properties of powder adsorbents based on iron oxides in form of finely-dispersed iron-ore concentrate (magnetite). To reduce the bulk weight of the complex adsorbent, slightly reduce the magnetic susceptibility and to increase the adsorption properties, as a sorption component there was chosen disintegrating electric-furnace steelmaking slag (hereafter referred to as slag) obtained by the dry air-cooling of melt, discharged from a steelmaking unit [8].
The components were selected according to the presence of iron-containing phases and the similarity of qualitative chemical compositions for the stronger bond formation at the intermolecular interactions.
In its chemical composition, slag (tab.1) contains predominantly CaО (45%) and SiO2 (59%), which determines its adsorbability to oil products, and the presence of iron oxides contributes to the demonstration of magnetic properties. The predominant content of iron oxides (Fe2O + Fe2O3 = 94,6 %) in magnetite (tab.2) indicates its high magnetic susceptibility. The components dispersity amounted to less than 100 µm.
The chemical composition of the adsorbent components was determined with an X-ray fluorescent spectrometer ARL 9900 (tab. 1,2).
Table 1. The chemical composition of electric-furnace steelmaking slag (w/w %)
The research was carried out for each component separately and for their mixture at the optimum ratio.
The ratio of the components was chosen according to the conditions of optimal magnetic susceptibility, measured with the Faraday method by the readings of torsion balance in magnetic field. The maximum magnetic field intensity of the coil was 18 kА/m. The optimum mass ratio was determined at 40% of adsorbing component and 60% of magnetic filler (1:1,5), which corresponded to the virtually equal volume ratio of components.
To provide floatability and reduce water sorption the complex adsorbent was modified with silicone water repellant (tab. 3) [9].
Table 3. The technical characteristics of “YARKO” water repellant
|
Medium reaction (рН of water extract) |
11,5–13 |
|
Water sorption (% by weight) |
3 |
|
Density (kg/m3) |
1020 |
|
Solid residue, no more than (%) |
1,8 |
YARKO is a water-repellent aqueous-based organosilicon fluid, colorless and odourless. It is non-toxic and non-combustible, doesn’t contain organic solvents, forms no film, doesn’t prevent the air circulation, and doesn’t alter the air and vapor permeability of materials.
As a result of modifying the material it was identified that the necessary amount of water repellant for achieving the maximum floatability (3 h) is 8-10%. The water sorption of samples amounts to 6-10%.
The water sorption data is confirmed with the values of contact angle of wetting for a water drop, placed on the surface of the compacted material layer, which amounted to 115-1200 (fig. 1).
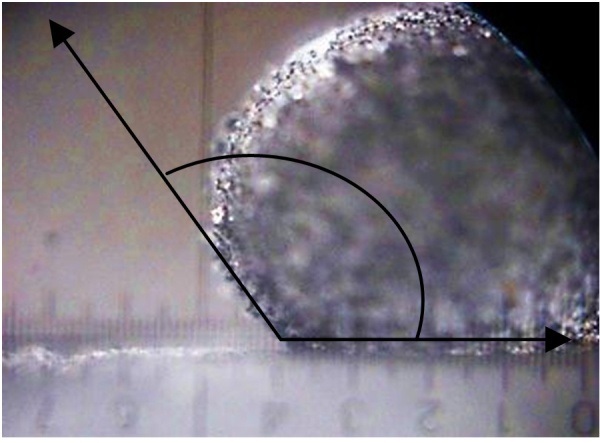
Fig. 1. Water drop on the surface of complex adsorbent layer(enlargement ratio – 50).
The alteration of contact angle of wetting for oil products from the highest value 1200 to 0 after placing an oil drop on the compacted layer of complex adsorbent took place in 6-10 seconds, which proves the rapid oil saturation into the adsorbent layer.
The values of weight oil capacity of the complex adsorbent under research were from 0,6 to 1 kg/kg (depending on the type of oil products). At this, the volume oil capacity amounted to values from 960 to
Table 4. Comparative characteristics of the used industrial adsorbents and the suggested adsorbent
|
№ |
Adsorbent |
Bulk weight, g/m3 |
Weight oil capacity, kg/kg |
Oil capacity for |
|
1 |
Saw-dust |
150 |
5 |
750 |
|
2 |
Lesorb-extra |
60 |
10 |
600 |
|
3 |
Sorboil А |
370 |
3 |
1110 |
|
4 |
Peat |
100-200 |
5-3 |
500-1000 |
|
Suggested |
Iron-ore concentrate +slag |
1610 |
1,0 |
1610 |
From the given characteristics we can make a conclusion that the developed adsorbent has the highest volume oil capacity, which is useful at its transportation by oil recovery vessels with the limited stowage space for cargo.
The suggested magnetic adsorbent is flameproof and explosion-proof, reusable, manufacturable and cost-effective.
The oil products are cleaned up from the water surface according to the scheme, presented in fig. 2 [10].
.jpg)
From the hopper 9 the adsorbent is distributed about the surface of the treated water. Due to the increased bulk weight the adsorbent saturation time amounts to 6-10 seconds, within which, due to the vessel’s movement, it reaches the oil-gathering drum 4, being on the water surface. The large non-floating flocculi sedimentate on the moving belt 3 and are also moved to the oil-gathering drum, where they are attracted to its surface with the magnetic field. The saturated adsorbent is removed from the drum surface with a scraper 5. The gathered mix is removed with a rotating auger 7 to the receptacle 8.
The gathered oil products are separated in a scroll centrifuge in steady-state conditions.
III. Conclusion
So, the suggested facility allows the efficient gathering of viscous oil products with the increased bulk weight adsorbents due to their rapid sinking into the oil-products layer, which can’t be achieved with light adsorbents. The full saturation of adsorbent is provided with the vessel speed. To provide the contact time of adsorbent with spilled oil products within 10 seconds, and with the distance of
IV. Inference
The presented findings of the research demonstrate that the use of magnetic adsorbents together with the magnetic field effect on oil products provides the considerable positive effect at the appropriate engineering design of the used facilities.
The usage of magnetic adsorbents with bulk weight, which exceeds the oil products density, provides their rapid magnetizing and the efficient extraction with magnetic separators.
The article is prepared within the framework of implementing the project part of the state task of Assignment № 14.2406.2014/К.
1. S. Wang and Y. Peng, Natural zeolites as effective adsorbents in water and wastewater treatment. Chemical Engineering Journal, vol.156, 2010, pp. 11-24.
2. H. Zhou and D.W. Smith, Advanced technologies in water and wastewater treatment. J. Environ, vol.1, 2002, pp. 247-264.
3. M.A. Malik, A. Ghaffar and S.A. Malik, Water purification by electrical discharges. Plasma Sources Sci. Technol, vol.10, 2001. pp. 82-91.
4. N.P. Solntseva, and O.A. Guseva, Distribution of oil and soil products in soils of tundra landscapes within the European territory of Russian. Proc. Intern. Symp. of physics, chemistry and ecology of seasonally frozen soils, Alaska, 1997, pp. 449-455.
5. J.A. Fay, Physical processes in the spread of oil on a water surface. Proceeding of the Joint Conference on the Prevention and Control of Oil Spills, American Petroleum Institute, 1971. pp: 463-467.
6. O.S. Mochalova, L.M. Gurvich and N.M. Antonova, “Methods of dealing with accidental pollution of water bodies with oil,” Environmental protection in the oil and gas sector, vol.3, 2004, pp. 20 -25.
7. O.V. Kravchenko, V.V. Lapko and D.I. Shvets, Using sorbents based on plant raw material for extracting oil products from water environment and alkali-oil emulsions. Moscow: Chemistry, 1995.
8. Yu.E. Tokach, Yu.K. Rubanov, A.S. Ivanov and I.I. Arkatova, “Somposite sorbents based on iron oxides to extract hydrocarbons from aqueous media,” Fundamental research. vol. 11 (8), 2014, pp. 1692- 1697.
9. Yu.E. Tokach and Yu.K. Rubanov, Composite sorbents based on iron oxides for coolant-cutting fluids recycling, monograph. BSTU publishing office, Belgorod, 2014.
10. Patent of RF 2535744 (2014). Yu.K. Rubanov and Yu.E. Tokach. Device for collecting oil from the water surface.







